Is Hbr An Acid Or Base
listenit
Mar 23, 2025 · 5 min read
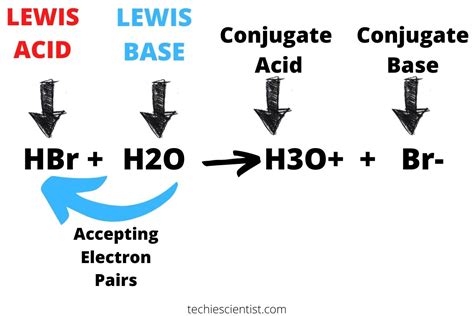
Table of Contents
Is HBr an Acid or Base? A Deep Dive into Hydrogen Bromide
Hydrogen bromide (HBr), a colorless gas at room temperature with a pungent, irritating odor, is a crucial chemical compound with significant applications in various industries. However, a fundamental question often arises regarding its chemical nature: Is HBr an acid or a base? The answer, as we'll explore in detail, is unequivocally: HBr is a strong acid. This article will delve into the reasons behind this classification, examining its properties, behavior in aqueous solutions, and its applications.
Understanding Acids and Bases
Before delving into the specifics of HBr, let's establish a clear understanding of acids and bases. Several theories explain acid-base behavior, but the most relevant for understanding HBr is the Brønsted-Lowry theory. This theory defines an acid as a proton (H⁺) donor and a base as a proton acceptor.
A strong acid is one that completely dissociates in water, releasing all its protons. Conversely, a weak acid only partially dissociates. The strength of an acid is determined by its dissociation constant (Ka). A higher Ka value indicates a stronger acid. Bases are similarly classified as strong or weak depending on their degree of dissociation.
The Case for HBr as a Strong Acid
HBr's acidic nature stems from its ability to readily donate a proton when dissolved in water. The dissociation reaction is as follows:
HBr(g) + H₂O(l) → H₃O⁺(aq) + Br⁻(aq)
This equation shows that when HBr dissolves in water, it completely dissociates into hydronium ions (H₃O⁺) – the hydrated form of protons – and bromide ions (Br⁻). The complete dissociation is the hallmark of a strong acid. The presence of a high concentration of H₃O⁺ ions is what makes the solution acidic, leading to a low pH value. This is why solutions of HBr are highly acidic.
Examining the Bond Strength
The strength of HBr as an acid is also linked to the bond strength between the hydrogen and bromine atoms. The H-Br bond is relatively weak compared to bonds in other hydrogen halides. This weaker bond allows for easier dissociation of the proton, further contributing to its strong acidic nature. The electronegativity difference between hydrogen and bromine, while significant, isn't the sole determining factor; the bond strength plays a crucial role.
Comparing HBr to Other Hydrogen Halides
HBr belongs to the group of hydrogen halides (HF, HCl, HBr, HI). All hydrogen halides except HF are strong acids. This trend reflects the decreasing bond strength as we move down the halogen group in the periodic table. The large size of the bromine atom means the H-Br bond is weaker than the H-F bond, allowing for easier proton donation.
- HF (Hydrofluoric acid): A weak acid due to the strong H-F bond.
- HCl (Hydrochloric acid): A strong acid.
- HBr (Hydrobromic acid): A strong acid, even stronger than HCl.
- HI (Hydroiodic acid): A strong acid, the strongest among the hydrogen halides.
Applications of Hydrobromic Acid
The strong acidic nature of HBr makes it a versatile reagent in various applications:
1. Industrial Processes:
- Production of Alkyl Bromides: HBr is used to produce alkyl bromides, which are essential intermediates in organic synthesis. These compounds are used in pharmaceuticals, pesticides, and other chemical products.
- Metal Cleaning: Its strong acidic properties make it effective in cleaning metals and removing oxides from metal surfaces.
- Petroleum Refining: HBr plays a role in some petroleum refining processes.
2. Laboratory Applications:
- Acid-Catalyzed Reactions: In laboratories, HBr is frequently used as a catalyst in various acid-catalyzed reactions in organic chemistry. Its high acidity makes it effective for reactions requiring a strong acid catalyst.
- Synthesis of Inorganic Compounds: It's used in the synthesis of various inorganic compounds.
- Analytical Chemistry: HBr finds applications in analytical chemistry procedures.
3. Other Applications:
- Medicine: While not directly used as a medicine, HBr's compounds have medicinal applications.
- Photography: Certain bromides derived from HBr have applications in photography.
Safety Precautions when Handling HBr
HBr is a highly corrosive and reactive substance. Therefore, handling it requires stringent safety precautions:
- Protective Gear: Always wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat. A respirator may be necessary when handling gaseous HBr.
- Ventilation: Work in a well-ventilated area or use a fume hood to avoid inhaling the fumes, which are extremely irritating to the respiratory system.
- Proper Storage: Store HBr in a tightly sealed container in a cool, dry place away from incompatible materials.
- Emergency Procedures: Be aware of the emergency procedures in case of spills or accidents.
Conclusion: HBr – A Definitive Strong Acid
In summary, HBr is unequivocally a strong acid. Its complete dissociation in water, the relatively weak H-Br bond strength, and its consequent high concentration of hydronium ions all solidify its classification as a strong acid. Its strong acidic properties make it a valuable reagent in various industrial and laboratory settings. However, its corrosive nature necessitates careful handling and adherence to strict safety protocols. Understanding its properties and applications is crucial for its safe and effective use in various fields. Further research into its behavior under different conditions and its interactions with other compounds can contribute to the advancement of chemistry and its diverse applications. The strength of HBr as an acid firmly establishes its important role in the broader context of chemistry.
Latest Posts
Related Post
Thank you for visiting our website which covers about Is Hbr An Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
