How To Calculate Mass Of A Liquid
listenit
Mar 23, 2025 · 7 min read
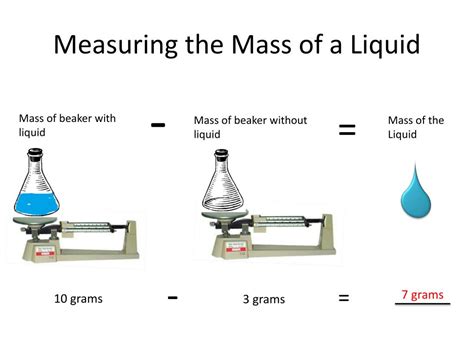
Table of Contents
How to Calculate the Mass of a Liquid: A Comprehensive Guide
Determining the mass of a liquid is a fundamental task in various scientific disciplines, from chemistry and physics to engineering and environmental science. While seemingly simple, accurately calculating the mass requires understanding different methods and potential sources of error. This comprehensive guide will delve into various techniques for calculating the mass of a liquid, exploring both direct and indirect measurement methods, highlighting their strengths and weaknesses, and providing practical tips for accurate results.
Understanding Mass and its Relationship to Volume and Density
Before diving into the methods, let's establish the core relationship between mass, volume, and density. Mass is a measure of the amount of matter in an object or substance. Volume is the amount of space occupied by that matter. Density is the relationship between mass and volume, defined as mass per unit volume. This relationship is expressed by the following formula:
Density (ρ) = Mass (m) / Volume (V)
This formula is crucial because it allows us to calculate the mass of a liquid if we know its volume and density. Conversely, knowing the mass and volume allows us to calculate the density, a crucial property for identifying unknown substances.
Methods for Calculating the Mass of a Liquid
Several methods can be used to calculate the mass of a liquid, each with its own advantages and disadvantages:
1. Direct Measurement Using a Weighing Scale
This is the most straightforward and often the most accurate method. It involves directly weighing the liquid using a calibrated weighing scale. This requires:
- A suitable container: Choose a container that is clean, dry, and of known mass (tare weight). Beakers, Erlenmeyer flasks, or volumetric flasks are commonly used depending on the precision required.
- A calibrated weighing scale: The accuracy of the scale directly impacts the accuracy of the mass measurement. Analytical balances offer the highest precision, while top-loading balances are suitable for less demanding applications.
- Careful handling: Avoid spills or splashes during the weighing process. Ensure the container is stable on the weighing scale.
Procedure:
- Tare the scale: Place the empty container on the scale and zero it (tare function). This subtracts the container's mass from subsequent measurements.
- Add the liquid: Carefully transfer the liquid into the container, avoiding spills.
- Record the mass: Record the mass displayed on the scale. This value directly represents the mass of the liquid.
Advantages: This is a simple, direct, and often highly accurate method.
Disadvantages: It can be time-consuming for multiple samples, requires a calibrated scale, and might be impractical for very large volumes of liquids.
2. Calculating Mass from Volume and Density
If the volume and density of the liquid are known, the mass can be easily calculated using the density formula:
Mass (m) = Density (ρ) × Volume (V)
This method requires:
- Accurate volume measurement: Use appropriate measuring instruments like graduated cylinders, volumetric pipettes, or burettes, depending on the required precision. Consider the meniscus when reading the volume.
- Known density: The density of the liquid must be known or readily available from reference sources. Temperature can significantly affect density, so ensure the density value corresponds to the liquid's temperature.
Procedure:
- Measure the volume: Carefully measure the volume of the liquid using an appropriate measuring instrument.
- Obtain the density: Find the density of the liquid from a reliable source, considering the temperature.
- Calculate the mass: Substitute the volume and density values into the formula: Mass (m) = Density (ρ) × Volume (V).
Advantages: This is a convenient method when direct weighing is impractical or impossible.
Disadvantages: The accuracy depends heavily on the accuracy of the volume and density measurements. Incorrect density values due to temperature differences can lead to significant errors.
3. Using a Hydrometer
A hydrometer is a simple instrument used to measure the density of liquids. Once the density is determined, the mass can be calculated using the method described above. Hydrometers are particularly useful for measuring the density of solutions, such as battery acid or antifreeze. The specific gravity reading provided by a hydrometer can be converted to density using the density of water as a reference.
Procedure:
- Float the hydrometer: Carefully float the hydrometer in the liquid sample.
- Read the scale: Note the reading on the hydrometer's scale, which corresponds to the specific gravity or density of the liquid.
- Calculate density (if specific gravity is given): Density = Specific Gravity × Density of water (usually 1 g/mL at 4°C).
- Calculate mass: Use the calculated density and the measured volume to calculate the mass using the formula: Mass (m) = Density (ρ) × Volume (V).
Advantages: Hydrometers are simple and inexpensive tools for density measurement, especially useful for field measurements.
Disadvantages: They are not as precise as other methods, and the accuracy can be affected by temperature and other factors.
4. Advanced Techniques: Archimedes' Principle and Displacement Method
Archimedes' principle states that the buoyant force on an object submerged in a fluid is equal to the weight of the fluid displaced by the object. This principle can be used to determine the mass of a liquid indirectly.
This method requires:
- A known mass object (e.g., a sinker): An object of known mass that will sink in the liquid.
- A graduated cylinder: To measure the volume of liquid displaced.
- Accurate weighing scale: To measure the mass of the sinker.
Procedure:
- Measure the sinker's mass: Weigh the sinker using a calibrated scale.
- Measure the initial volume of the liquid: Fill a graduated cylinder with the liquid and note the volume.
- Submerge the sinker: Carefully submerge the sinker in the liquid, ensuring it doesn't touch the sides or bottom.
- Measure the final volume: Note the new volume of the liquid with the submerged sinker.
- Calculate the displaced volume: Subtract the initial volume from the final volume. This is the volume of liquid displaced by the sinker, which is equal to the volume of the sinker submerged.
- Calculate the density of the liquid: Density = mass of the sinker / Volume of liquid displaced. Note that this only works if the sinker is fully submerged.
- Calculate the mass of the liquid: Multiply the density by the total volume of liquid in the cylinder.
Advantages: A useful method when direct mass measurement is challenging.
Disadvantages: It's a less precise method compared to direct weighing, and the accuracy depends on the precision of volume measurements and the known mass of the sinker. This method becomes unreliable if the sinker is not fully submerged.
Sources of Error and Minimization Strategies
Several factors can contribute to errors in mass calculation:
- Inaccurate weighing scale: Ensure the scale is properly calibrated and maintained.
- Improper volume measurement: Use appropriate measuring instruments and carefully read the meniscus.
- Temperature variations: Temperature affects the density of liquids. Control the temperature or use density values corresponding to the actual temperature.
- Evaporation: For volatile liquids, evaporation can lead to mass loss during measurement. Minimize exposure time or use sealed containers.
- Spills and splashes: Avoid spills and splashes during handling to ensure accurate volume and mass measurements.
- Incomplete mixing: For solutions, ensure thorough mixing before sampling to obtain a representative sample.
Minimizing these errors requires careful technique, precise instruments, and attention to detail. Repeating measurements and taking averages can help reduce random errors.
Choosing the Right Method
The best method for calculating the mass of a liquid depends on several factors, including:
- Accuracy required: For high-precision measurements, direct weighing is preferred. For less demanding applications, calculations based on volume and density might suffice.
- Available equipment: The choice of method is limited by the available instruments.
- Nature of the liquid: Volatile liquids require special precautions to minimize evaporation.
- Sample size: Direct weighing might be impractical for very large volumes.
Careful consideration of these factors will help in selecting the most appropriate and effective method for calculating the mass of a liquid.
Conclusion
Calculating the mass of a liquid is a fundamental task with various applications across different fields. This guide has explored multiple methods, including direct weighing, calculation using density and volume, hydrometer usage, and the Archimedes' principle. Each method possesses unique advantages and disadvantages, and the selection should be tailored based on the specific requirements and available resources. By understanding the underlying principles and potential sources of error, one can accurately determine the mass of a liquid and ensure the reliability of experimental results. Remember to always prioritize precision and accuracy in your measurements to achieve reliable and meaningful results. Careful technique and the appropriate choice of method are key to obtaining accurate results.
Latest Posts
Latest Posts
-
27 To The Power Of 2 3
Mar 24, 2025
-
How Did Reza Pahlavi Differ From Ayatollah Khomeini
Mar 24, 2025
-
Least Common Multiple Of 10 And 14
Mar 24, 2025
-
What Is The Lcm For 10 And 8
Mar 24, 2025
-
How Many Quarts Is 7 Pints
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Mass Of A Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
