What Is The Electron Configuration For Lithium
listenit
Mar 25, 2025 · 5 min read
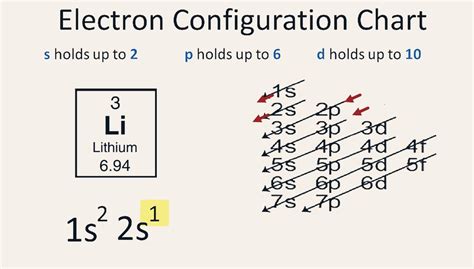
Table of Contents
What is the Electron Configuration for Lithium? A Deep Dive into Atomic Structure
Lithium, the lightest of the alkali metals, holds a special place in the periodic table and in the world of chemistry. Understanding its electron configuration is key to unlocking its unique properties and reactivity. This comprehensive guide will delve into the electron configuration of lithium, exploring its implications for chemical bonding, reactivity, and its place within the broader context of atomic structure.
Understanding Electron Configuration
Before we dive into lithium's specific configuration, let's establish a foundational understanding of what electron configuration represents. Electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. It dictates how an atom will interact with other atoms, forming chemical bonds and influencing its chemical properties.
This arrangement follows specific rules, governed by the principles of quantum mechanics:
- Aufbau Principle: Electrons fill the lowest energy levels first.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, with opposite spins.
- Hund's Rule: Electrons will individually occupy each orbital within a subshell before pairing up.
These principles guide us in predicting the electron configuration of any element, including lithium.
Determining the Electron Configuration of Lithium (Li)
Lithium (Li) has an atomic number of 3, meaning it possesses three protons in its nucleus and, in its neutral state, three electrons surrounding the nucleus. To determine its electron configuration, we'll follow the Aufbau principle and the rules mentioned above.
The lowest energy level is the first shell (n=1), which contains only the s subshell. This s subshell can hold a maximum of two electrons. Therefore, the first two electrons of lithium fill this 1s orbital.
The next energy level is the second shell (n=2), which contains both s and p subshells. The 2s subshell can also hold up to two electrons. The remaining electron of lithium occupies this 2s orbital.
Therefore, the complete electron configuration of lithium is: 1s²2s¹.
This concise notation tells us that:
- Two electrons are in the 1s orbital (closest to the nucleus).
- One electron is in the 2s orbital (further from the nucleus).
Visualizing the Electron Configuration of Lithium
A visual representation can further solidify our understanding. We can use orbital diagrams to depict the electron configuration:
1s: ↑↓
2s: ↑
Each arrow represents an electron, and the upward and downward arrows signify opposite spins. The filled 1s orbital shows the two electrons paired, while the single arrow in the 2s orbital indicates an unpaired electron. This unpaired electron is crucial in understanding lithium's reactivity.
Lithium's Reactivity and its Electron Configuration
The presence of that single unpaired electron in the 2s orbital is the key to understanding lithium's high reactivity. Atoms strive for stability, often achieved by having a full outermost electron shell (octet rule). Lithium, with its single valence electron, readily loses this electron to achieve a stable electron configuration similar to helium (1s²).
This electron loss results in the formation of a positively charged lithium ion (Li⁺), readily participating in ionic bonding with electronegative elements like chlorine (Cl) to form lithium chloride (LiCl). The strong electrostatic attraction between the Li⁺ ion and the Cl⁻ ion holds the compound together.
Comparison with Other Alkali Metals
Lithium belongs to Group 1 of the periodic table, the alkali metals. All alkali metals share the common characteristic of having a single valence electron in their outermost s subshell. This similarity leads to consistent trends in their properties:
- Low Ionization Energy: They readily lose their valence electron, resulting in low ionization energies.
- High Reactivity: Their tendency to lose electrons makes them highly reactive, especially with water and halogens.
- Formation of +1 Ions: They consistently form +1 ions.
However, lithium displays some unique properties compared to heavier alkali metals due to its small atomic size and high charge density. These differences manifest in its melting point, reactivity with water, and solubility of its salts.
Lithium's Applications: A Consequence of its Electron Configuration
The unique electron configuration of lithium and its subsequent properties lead to a wide range of applications:
- Batteries: Lithium-ion batteries, widely used in portable electronics and electric vehicles, leverage lithium's ability to readily lose and gain electrons.
- Lubricants: Lithium-based greases are used as high-temperature lubricants because of lithium's ability to form stable complexes.
- Ceramics and Glass: Lithium compounds are added to ceramics and glass to improve their properties, such as strength and thermal resistance.
- Medicine: Lithium salts have been used in the treatment of certain mental illnesses.
Advanced Concepts and Further Exploration
While the 1s²2s¹ configuration provides a basic understanding, more sophisticated models are necessary for a complete description of lithium's electronic structure.
- Quantum Mechanical Models: More accurate models, like Hartree-Fock or Density Functional Theory calculations, provide a more detailed picture of the electron distribution and energy levels. These models account for electron-electron repulsion and other subtle effects not captured in the simple orbital diagrams.
- Excited States: When lithium absorbs energy, its electron can jump to a higher energy level. These excited states are important in understanding lithium's spectroscopic properties and its behavior in various chemical reactions.
Conclusion: The Significance of Lithium's Electron Configuration
The seemingly simple electron configuration of lithium (1s²2s¹) is the foundation for understanding its unique chemical and physical properties. Its single valence electron dictates its high reactivity, ability to form ionic compounds, and its widespread applications in various technological fields. By understanding the fundamental principles of electron configuration and applying them to lithium, we gain valuable insights into the behavior of this essential element and its crucial role in our modern world. Further exploration into advanced quantum mechanical models provides even deeper understanding of its nuanced behavior and potential for future applications. The study of lithium's electron configuration serves as a gateway to appreciating the complexities and beauty of atomic structure and its profound impact on the macroscopic world around us.
Latest Posts
Latest Posts
-
How To Find Number Of Core Electrons
Mar 28, 2025
-
What Is The Formula For The Compound Magnesium Oxide
Mar 28, 2025
-
What Is The Correct Formula For Calcium Oxide
Mar 28, 2025
-
What Is The Si Base Unit Of Length
Mar 28, 2025
-
What Is The Oxidation State Of Each Element In Coh2
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Lithium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
