What Is The Electron Arrangement For Potassium
listenit
Mar 27, 2025 · 6 min read
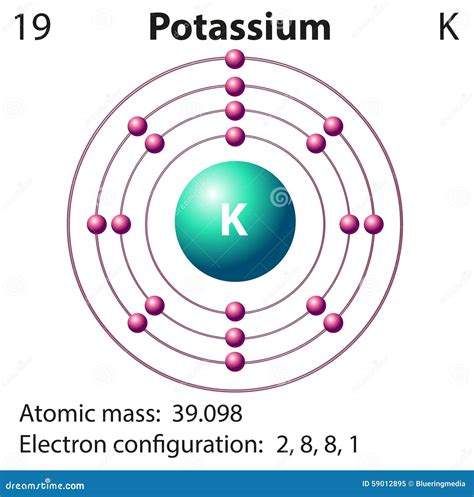
Table of Contents
What is the Electron Arrangement for Potassium? A Deep Dive into Atomic Structure
Potassium, a vital element for human health and a common component in various chemical reactions, holds a fascinating place in the periodic table. Understanding its electron arrangement is crucial to grasping its chemical properties and reactivity. This article provides a comprehensive exploration of potassium's electron configuration, delving into the underlying principles of atomic structure and demonstrating its significance in chemistry and beyond.
Understanding Electron Configuration
Before we dive into the specifics of potassium's electron arrangement, let's establish a foundational understanding of what electron configuration represents. The electron configuration of an atom describes how electrons are distributed among the various energy levels and sublevels within the atom. This distribution dictates an atom's chemical behavior, determining its reactivity and the types of bonds it can form.
The arrangement follows specific rules, primarily governed by the Aufbau principle, which states that electrons fill orbitals from the lowest energy level to the highest. The Pauli exclusion principle dictates that each orbital can hold a maximum of two electrons with opposite spins. Finally, Hund's rule suggests that electrons will individually occupy each orbital within a subshell before pairing up.
These principles, in conjunction with the quantum mechanical model of the atom, allow us to predict and understand the electron configuration of any element, including potassium.
Potassium's Position in the Periodic Table
Potassium (K), with an atomic number of 19, is located in Group 1 (alkali metals) and Period 4 of the periodic table. Its position provides valuable clues about its electron configuration. Being in Group 1 indicates it possesses one valence electron – an electron in its outermost shell. This single valence electron plays a pivotal role in potassium's reactivity. Its placement in Period 4 suggests that its electrons occupy four principal energy levels (shells).
Determining Potassium's Electron Configuration
Given potassium's atomic number of 19, it possesses 19 electrons. Using the Aufbau principle, we systematically fill the energy levels and sublevels:
- 1s²: The first energy level (n=1) contains only the 's' subshell, which can hold a maximum of two electrons.
- 2s² 2p⁶: The second energy level (n=2) contains an 's' subshell (holding two electrons) and a 'p' subshell (holding six electrons).
- 3s² 3p⁶: The third energy level (n=3) also consists of an 's' subshell (two electrons) and a 'p' subshell (six electrons).
- 4s¹: Finally, the fourth energy level (n=4) begins to fill with one electron in the 's' subshell.
Therefore, the full electron configuration of potassium is 1s²2s²2p⁶3s²3p⁶4s¹.
This configuration concisely summarizes the distribution of potassium's 19 electrons across its various energy levels and sublevels.
Significance of the 4s¹ Electron
The single electron in the 4s orbital is potassium's valence electron. This outermost electron is loosely held and readily participates in chemical reactions. Potassium's high reactivity stems directly from this easily lost valence electron. This explains why potassium readily forms a +1 ion (K⁺) by losing this electron to achieve a stable electron configuration, mimicking the noble gas argon (Ar).
Potassium's Chemical Properties and Reactivity
Potassium's electron configuration directly dictates its chemical properties:
- High Reactivity: The readily available valence electron makes potassium highly reactive, particularly with water and other oxidizing agents. Reactions with water are often vigorous and exothermic, producing hydrogen gas and potassium hydroxide.
- Electropositivity: Potassium is highly electropositive, meaning it readily loses its valence electron to form a positive ion. This propensity to lose electrons is a defining characteristic of alkali metals.
- Formation of Ionic Compounds: Potassium's tendency to lose an electron leads to the formation of ionic compounds with non-metals. The electrostatic attraction between the positively charged potassium ion (K⁺) and negatively charged non-metal ions forms strong ionic bonds. Examples include potassium chloride (KCl) and potassium oxide (K₂O).
- Reducing Agent: Due to its ease of losing electrons, potassium acts as a powerful reducing agent in many chemical reactions, donating electrons to other substances.
Applications of Potassium and its Compounds
Potassium's unique chemical properties lead to diverse applications across various fields:
- Fertilizers: Potassium is a crucial macronutrient for plant growth, essential for various metabolic processes. Potassium-containing fertilizers significantly enhance agricultural productivity.
- Medicine: Potassium plays a vital role in maintaining electrolyte balance in the human body, influencing nerve impulses and muscle contractions. Potassium supplements are used to address potassium deficiencies.
- Industrial Applications: Potassium compounds find applications in the manufacturing of glass, soap, and various other industrial products. Potassium hydroxide (KOH) is a strong base used in many chemical processes.
- Food Science: Potassium is a crucial component in many food products, contributing to their flavor and texture. Potassium chloride (KCl) serves as a salt substitute in low-sodium diets.
Orbital Diagrams and Electron Spin
While the electron configuration provides an overall picture, a more detailed representation involves orbital diagrams. These diagrams illustrate the individual orbitals within each subshell and the arrangement of electrons within them, including their spins.
For example, the orbital diagram for potassium would show:
- Two electrons in the 1s orbital (opposite spins)
- Two electrons in the 2s orbital (opposite spins)
- Six electrons in the 2p orbitals (three pairs with opposite spins)
- Two electrons in the 3s orbital (opposite spins)
- Six electrons in the 3p orbitals (three pairs with opposite spins)
- One electron in the 4s orbital (unpaired spin).
This level of detail helps visualize the electron distribution within the atom and clarifies the concept of electron spin.
Exceptions to the Aufbau Principle
While the Aufbau principle generally predicts electron configurations accurately, some exceptions exist, especially in transition metals and inner transition metals. These exceptions arise from the close proximity of energy levels and the stabilizing effects of half-filled and fully filled subshells. However, potassium’s electron configuration neatly follows the Aufbau principle, showcasing its straightforward electronic structure.
Advanced Concepts: Quantum Numbers and Atomic Orbitals
A deeper understanding of electron arrangement involves quantum numbers and atomic orbitals. Each electron in an atom is described by four quantum numbers:
- Principal quantum number (n): Specifies the energy level (shell).
- Azimuthal quantum number (l): Specifies the subshell (s, p, d, f).
- Magnetic quantum number (ml): Specifies the orbital within the subshell.
- Spin quantum number (ms): Specifies the spin of the electron (+1/2 or -1/2).
These quantum numbers provide a precise description of an electron's state within the atom and are essential in explaining the details of atomic structure and electron behavior. The shapes and energies of atomic orbitals are directly related to these quantum numbers, defining the probability of finding an electron in a specific region of space around the nucleus.
Conclusion: Potassium's Electron Arrangement and its Chemical Significance
Potassium's electron arrangement, specifically its single valence electron, is the key to understanding its reactivity and chemical behavior. This single electron readily participates in chemical reactions, explaining potassium's role as a highly reactive alkali metal and its widespread use in various applications. The principles of atomic structure, including the Aufbau principle, Pauli exclusion principle, Hund's rule, and quantum numbers, provide the theoretical framework for understanding and predicting the electron arrangement of all elements, including potassium. Understanding this arrangement is fundamental to appreciating potassium's unique properties and its crucial role in both the natural world and technological advancements. From fertilizers to medicine, the seemingly simple electron configuration of potassium underpins its significant contribution to numerous fields.
Latest Posts
Latest Posts
-
Are Frequency And Wavelength Directly Proportional
Mar 30, 2025
-
What Is The Fraction For 40
Mar 30, 2025
-
What Is 8 Divided By 7
Mar 30, 2025
-
What Is The Length Of In The Right Triangle Below
Mar 30, 2025
-
How Far Is Jupiter From The Sun In Astronomical Units
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Arrangement For Potassium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
