What Is The Conjugate Acid Of Oh
listenit
Mar 25, 2025 · 6 min read
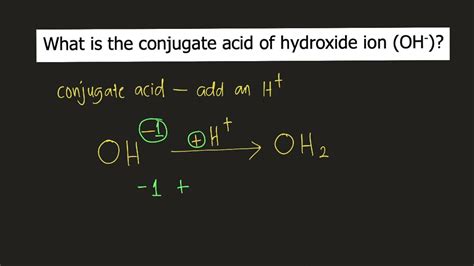
Table of Contents
What is the Conjugate Acid of OH⁻? Understanding Acid-Base Chemistry
The question, "What is the conjugate acid of OH⁻?" delves into the fundamental concepts of acid-base chemistry, specifically the Brønsted-Lowry theory. Understanding conjugate acid-base pairs is crucial for predicting reaction outcomes and manipulating chemical equilibria. This comprehensive article will explore the conjugate acid of hydroxide (OH⁻), examining its properties, formation, and relevance in various chemical contexts. We'll also discuss related concepts to solidify your understanding of acid-base chemistry.
Understanding Brønsted-Lowry Acid-Base Theory
Before diving into the specific conjugate acid of OH⁻, let's revisit the Brønsted-Lowry theory. This theory defines an acid as a proton (H⁺) donor and a base as a proton acceptor. Crucially, this theory highlights the conjugate relationship between acids and bases.
When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs are always linked; they differ only by a single proton (H⁺).
Identifying the Conjugate Acid of OH⁻
The hydroxide ion (OH⁻) is a strong base. It readily accepts a proton (H⁺). To find its conjugate acid, we simply add a proton to its structure:
OH⁻ + H⁺ → H₂O
Therefore, the conjugate acid of OH⁻ is water (H₂O).
This seemingly simple reaction illustrates a fundamental principle: the conjugate acid of a base is always one proton richer than the original base.
Properties of Water as the Conjugate Acid of OH⁻
Water, as a conjugate acid, exhibits amphoteric behavior. This means it can act as both an acid and a base, depending on the reaction environment.
-
As an acid: Water can donate a proton to a stronger base, forming the hydroxide ion (OH⁻). For instance, in a reaction with ammonia (NH₃), water acts as an acid:
H₂O + NH₃ ⇌ NH₄⁺ + OH⁻
-
As a base: Water can accept a proton from a stronger acid, forming the hydronium ion (H₃O⁺). For instance, in a reaction with hydrochloric acid (HCl):
H₂O + HCl → H₃O⁺ + Cl⁻
This amphoteric nature is crucial in many chemical processes and explains water's role as a solvent in many acid-base reactions.
The Importance of Conjugate Acid-Base Pairs in Equilibrium
Conjugate acid-base pairs play a pivotal role in chemical equilibria. The strength of an acid is directly related to the weakness of its conjugate base, and vice-versa. A strong acid has a very weak conjugate base, while a weak acid has a relatively strong conjugate base.
Consider the dissociation of a weak acid, HA:
HA ⇌ H⁺ + A⁻
In this equilibrium, HA is the acid, and A⁻ is its conjugate base. The position of the equilibrium depends on the relative strengths of HA and A⁻. If HA is a strong acid, the equilibrium lies far to the right, meaning most of the HA dissociates into H⁺ and A⁻. If HA is a weak acid, the equilibrium lies far to the left, meaning only a small fraction of HA dissociates.
Understanding this relationship between the acid and its conjugate base is crucial for predicting the behavior of acid-base systems and manipulating their equilibria.
Illustrative Examples and Applications
The concept of conjugate acids is not just a theoretical exercise; it has significant practical implications in various fields:
1. Buffer Solutions:
Buffer solutions are crucial in maintaining a stable pH. They typically consist of a weak acid and its conjugate base (or a weak base and its conjugate acid). The presence of both components allows the buffer to resist changes in pH upon the addition of small amounts of acid or base. For example, a common buffer system uses acetic acid (CH₃COOH) and its conjugate base, acetate (CH₃COO⁻).
2. Biological Systems:
Biological systems rely heavily on acid-base chemistry. Many biological molecules act as weak acids or bases, and their conjugate acid-base pairs play a critical role in maintaining physiological pH and enabling enzymatic reactions. For instance, the phosphate buffer system (H₂PO₄⁻/HPO₄²⁻) is essential for maintaining the pH of blood.
3. Industrial Processes:
Many industrial processes involve acid-base reactions, and understanding conjugate acid-base pairs is crucial for optimizing these processes. This includes processes such as water treatment, metal extraction, and the production of various chemicals.
4. Titration Curves:
Titration curves graphically represent the change in pH during a titration. The equivalence point, where the acid and base have completely reacted, is often associated with a significant change in pH. The presence of a conjugate acid or base influences the shape and characteristics of the titration curve.
5. Environmental Chemistry:
Understanding acid-base chemistry is also crucial in environmental studies. Acid rain, for example, results from the reaction of acidic pollutants with water in the atmosphere, leading to the formation of hydronium ions (H₃O⁺). The impact of acid rain on ecosystems involves changes in soil pH and water acidity, which affect plant and animal life.
Beyond the Basics: Delving Deeper into Acid-Base Strength
The strength of an acid or base determines the extent to which it donates or accepts protons. Strong acids and bases completely dissociate in water, while weak acids and bases only partially dissociate.
The strength of an acid is often indicated by its acid dissociation constant (Ka), while the strength of a base is indicated by its base dissociation constant (Kb). These constants are related through the ion product of water (Kw = Ka * Kb = 1.0 x 10⁻¹⁴ at 25°C).
A larger Ka value indicates a stronger acid, while a larger Kb value indicates a stronger base. This relationship highlights the inverse relationship between the strength of an acid and its conjugate base: a stronger acid has a weaker conjugate base, and vice-versa.
Advanced Concepts: Lewis Acid-Base Theory
While the Brønsted-Lowry theory is useful for many acid-base reactions, it doesn't encompass all types of acid-base interactions. The Lewis theory provides a broader definition: a Lewis acid is an electron-pair acceptor, and a Lewis base is an electron-pair donor.
This broader definition includes reactions that don't involve proton transfer, such as the reaction between boron trifluoride (BF₃) and ammonia (NH₃). BF₃ acts as a Lewis acid by accepting an electron pair from the nitrogen atom in NH₃, which acts as a Lewis base. Although this reaction doesn't involve proton transfer, it's still considered an acid-base reaction according to the Lewis definition.
Conclusion: The Significance of Conjugate Acid-Base Pairs
The conjugate acid of OH⁻, water (H₂O), is a cornerstone of understanding acid-base chemistry. Its amphoteric nature exemplifies the dynamic equilibrium that governs many chemical processes. The concept of conjugate acid-base pairs is fundamental to understanding buffer solutions, biological systems, industrial processes, titration analysis, and environmental chemistry. By mastering this concept, and expanding your knowledge to include the Lewis theory of acids and bases, you can achieve a comprehensive grasp of acid-base chemistry and its wide-ranging applications. This detailed exploration has highlighted the importance of understanding conjugate acid-base pairs in various chemical contexts, emphasizing their significance in both theoretical and practical applications. The ability to identify conjugate pairs and understand their properties is a crucial skill for success in chemistry and related fields.
Latest Posts
Latest Posts
-
What Subatomic Particles Make Up An Atom
Mar 27, 2025
-
How To Find Ph Of Buffer Solution
Mar 27, 2025
-
5 Less Than The Product Of 3 And A Number
Mar 27, 2025
-
Where Is Most Of Earths Freshwater Stored
Mar 27, 2025
-
How Many Protons Does Strontium Have
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Acid Of Oh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
