What Is The Conjugate Acid Of Hso4
listenit
Mar 28, 2025 · 6 min read
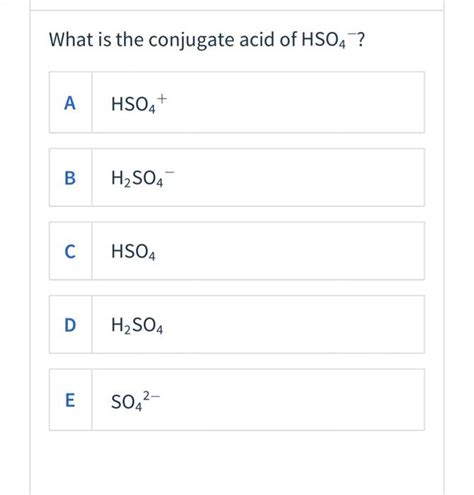
Table of Contents
What is the Conjugate Acid of HSO₄⁻? Understanding Brønsted-Lowry Theory
The question, "What is the conjugate acid of HSO₄⁻?" delves into the fundamental concepts of acid-base chemistry, specifically the Brønsted-Lowry theory. Understanding conjugate acid-base pairs is crucial for predicting reaction outcomes and grasping the behavior of acids and bases in various chemical systems. This article will comprehensively explore the conjugate acid of HSO₄⁻, explaining the underlying theory and providing relevant examples.
Brønsted-Lowry Acid-Base Theory: The Foundation
Before we identify the conjugate acid of HSO₄⁻, let's revisit the Brønsted-Lowry theory. This theory defines an acid as a proton (H⁺) donor and a base as a proton acceptor. Crucially, this theory highlights the concept of conjugate acid-base pairs. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs differ by only one proton (H⁺).
In simpler terms: Think of it like a seesaw. The acid on one side donates a proton, and the base on the other side accepts it. The resulting molecules are the conjugate pairs.
Identifying the Conjugate Acid of HSO₄⁻
HSO₄⁻, the bisulfate ion, acts as a weak base in certain reactions and a weak acid in others. This amphoteric nature means it can both donate and accept a proton, depending on the environment. To find its conjugate acid, we must consider what happens when it acts as a base and accepts a proton.
When HSO₄⁻ accepts a proton (H⁺), it forms H₂SO₄, sulfuric acid. Therefore, the conjugate acid of HSO₄⁻ is H₂SO₄.
HSO₄⁻ (base) + H⁺ (proton) ⇌ H₂SO₄ (conjugate acid)
This equilibrium highlights the reversible nature of acid-base reactions. The bisulfate ion can act as a base, accepting a proton to become sulfuric acid, but sulfuric acid can then donate a proton back, reforming the bisulfate ion.
Sulfuric Acid (H₂SO₄): A Strong Acid
H₂SO₄, sulfuric acid, is a well-known strong acid. This means it readily donates its protons in aqueous solutions. Its strength stems from the high electronegativity of the oxygen atoms and the stability of the bisulfate ion (HSO₄⁻) and sulfate ion (SO₄²⁻) formed after proton donation. The complete dissociation of sulfuric acid in water is represented as:
H₂SO₄(aq) → H⁺(aq) + HSO₄⁻(aq)
This first dissociation is essentially complete, while the second dissociation (HSO₄⁻ → H⁺ + SO₄²⁻) is less complete, reflecting the weaker acidic nature of the bisulfate ion.
Bisulfate Ion (HSO₄⁻): An Amphoteric Species
The amphoteric nature of HSO₄⁻ is crucial to understanding its behavior. It can act as both an acid and a base, depending on the solution it's in.
As an acid: In a solution with a strong base, HSO₄⁻ can donate a proton:
HSO₄⁻(aq) + OH⁻(aq) ⇌ SO₄²⁻(aq) + H₂O(l)
Here, HSO₄⁻ acts as an acid, donating a proton to the hydroxide ion (OH⁻), forming the sulfate ion (SO₄²⁻) and water. Its conjugate base in this reaction is SO₄²⁻.
As a base: In a solution with a strong acid, HSO₄⁻ can accept a proton:
HSO₄⁻(aq) + H⁺(aq) ⇌ H₂SO₄(aq)
As discussed previously, this reaction shows HSO₄⁻ acting as a base, accepting a proton from a strong acid to form sulfuric acid. Its conjugate acid is H₂SO₄.
Understanding Conjugate Acid-Base Pairs in Equilibrium
The equilibrium between an acid and its conjugate base (or a base and its conjugate acid) is described by the acid dissociation constant (Ka) for acids and the base dissociation constant (Kb) for bases. The relationship between Ka and Kb for a conjugate acid-base pair is given by:
Ka * Kb = Kw
where Kw is the ion product constant for water (approximately 1.0 x 10⁻¹⁴ at 25°C). This equation shows the inverse relationship between the strengths of a conjugate acid-base pair. A strong acid has a weak conjugate base, and a strong base has a weak conjugate acid.
Because sulfuric acid (H₂SO₄) is a strong acid, its conjugate base, the bisulfate ion (HSO₄⁻), is a relatively weak base. This doesn't mean it's not a base; it simply means its propensity to accept a proton is less than that of a strong base like hydroxide (OH⁻).
Practical Applications and Importance
Understanding conjugate acid-base pairs, especially the conjugate acid of HSO₄⁻ (H₂SO₄), has many practical applications across various fields. These include:
- Industrial processes: Sulfuric acid is a cornerstone chemical in many industrial processes, including fertilizer production, petroleum refining, and metal processing. Its properties as a strong acid are directly linked to its ability to readily donate protons.
- Analytical chemistry: The acid-base properties of HSO₄⁻ are exploited in titrations and other analytical techniques to determine the concentration of various substances.
- Environmental chemistry: Sulfuric acid plays a significant role in acid rain formation. Understanding its behavior in the atmosphere is crucial for mitigating its environmental impact.
- Biological systems: While less directly involved than in industrial processes, the principles of conjugate acid-base pairs are fundamental in understanding biological systems. Many biochemical reactions involve proton transfer, and the concepts of acidity and basicity are vital in understanding these processes.
Distinguishing between Acid and Conjugate Acid
It's crucial to emphasize the distinction between an acid and its conjugate acid. They are related but not interchangeable. An acid donates a proton to become its conjugate base. Its conjugate acid is formed when its conjugate base accepts a proton. The conjugate acid will always have one more proton than the original acid's conjugate base.
Examples of Other Conjugate Acid-Base Pairs
Understanding the concept of conjugate acid-base pairs extends beyond just HSO₄⁻ and H₂SO₄. Here are a few more examples:
- HCl (acid) and Cl⁻ (conjugate base): Hydrochloric acid donates a proton to become the chloride ion.
- NH₃ (base) and NH₄⁺ (conjugate acid): Ammonia accepts a proton to become the ammonium ion.
- H₂O (acid) and OH⁻ (conjugate base): Water can act as an acid, donating a proton to become the hydroxide ion.
- H₂CO₃ (acid) and HCO₃⁻ (conjugate base): Carbonic acid donates a proton to become the bicarbonate ion.
Conclusion: The Significance of Conjugate Acid-Base Pairs
The conjugate acid of HSO₄⁻, H₂SO₄, exemplifies the core concepts of Brønsted-Lowry acid-base theory. Understanding conjugate acid-base pairs is essential for predicting reaction pathways, interpreting experimental data, and comprehending the chemical behavior of various substances in different environments. The amphoteric nature of HSO₄⁻ further highlights the dynamic interplay between acids and bases and their pivotal role in countless chemical processes. The knowledge of conjugate acid-base pairs extends beyond theoretical concepts; it is crucial for practical applications across numerous scientific and industrial fields. By grasping this fundamental principle, we gain a more profound understanding of the intricate world of acid-base chemistry.
Latest Posts
Latest Posts
-
How Are 37 100 And 0 37 Equivalent
Mar 31, 2025
-
What Is The Density Of Rubbing Alcohol In Grams
Mar 31, 2025
-
27 4 As A Mixed Number
Mar 31, 2025
-
Is Burning A Match A Chemical Change
Mar 31, 2025
-
What Is The Difference Between A Solution And A Suspension
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Acid Of Hso4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
