Is Burning A Match A Chemical Change
listenit
Mar 31, 2025 · 5 min read
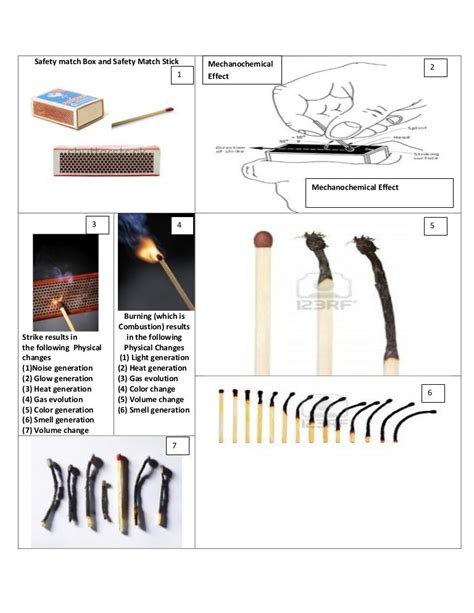
Table of Contents
Is Burning a Match a Chemical Change? A Deep Dive into Combustion
Burning a match seems like a simple act, a fleeting moment of flame. But beneath the surface of this everyday occurrence lies a complex process of chemical transformation. The question, "Is burning a match a chemical change?" has a resounding yes, and understanding why unlocks a deeper appreciation of chemistry and the world around us. This article will explore the chemical reactions involved in burning a match, the evidence supporting its classification as a chemical change, and some of the broader implications of this seemingly simple process.
Understanding Chemical Changes
Before delving into the specifics of a burning match, let's establish a clear understanding of what constitutes a chemical change. Unlike physical changes, which alter the form or appearance of a substance without changing its chemical composition (like melting ice or breaking a glass), chemical changes involve the creation of new substances with different chemical properties. These changes are often irreversible and are accompanied by observable phenomena such as:
- Formation of a gas: The release of bubbles or fumes.
- Formation of a precipitate: The creation of a solid from a solution.
- Color change: A noticeable shift in the hue of the substance.
- Temperature change: The release or absorption of heat (exothermic or endothermic reaction).
- Light emission: The production of light, often as a flame.
- Irreversibility: The inability to easily reverse the change back to the original state.
The Chemistry of a Burning Match
A match consists of several key components that contribute to its combustion:
-
The Match Head: This is the crucial part containing a mixture of chemicals designed to ignite readily. Common components include:
- Potassium chlorate (KClO₃): A strong oxidizing agent, supplying oxygen for the reaction.
- Red phosphorus (P): Reacts readily with the oxidizing agent.
- Sulfur (S): Lowers the ignition temperature, making it easier to ignite.
- Binder: Holds the components together.
- Filler: May include substances like powdered glass to increase friction.
-
The Matchstick: Typically made of wood, the matchstick acts as a fuel source once the match head ignites. Wood is primarily composed of cellulose, a complex carbohydrate.
-
The Striking Surface: Often composed of red phosphorus and a binder, this surface provides the necessary friction to initiate the reaction.
The Combustion Process: A Step-by-Step Breakdown
The act of striking a match initiates a chain of events that culminates in combustion:
-
Friction and Ignition: Striking the match head against the striking surface generates friction, producing heat. This heat triggers a reaction between the red phosphorus in the match head and the oxidizing agent (potassium chlorate).
-
Exothermic Reaction: This reaction is highly exothermic, meaning it releases a significant amount of heat. This heat is sufficient to ignite the sulfur and subsequently the wood of the matchstick.
-
Combustion of Sulfur: The sulfur ignites, producing more heat and contributing to the flame.
-
Combustion of Wood (Cellulose): The heat from the burning sulfur ignites the cellulose in the wood, which undergoes a rapid oxidation reaction with oxygen from the air. This reaction is also exothermic, producing heat, light, and various combustion byproducts.
Evidence of Chemical Change in Burning a Match
Several clear indicators demonstrate that burning a match is a chemical change:
-
Light and Heat Emission: The production of visible light (flame) and significant heat are strong indicators of an exothermic chemical reaction.
-
Gas Formation: The burning match produces gases like carbon dioxide (CO₂) and water vapor (H₂O), which are easily observable as smoke. These are new substances formed during the combustion process.
-
Irreversibility: Once the match has burned, it cannot be easily returned to its original state. The wood is charred and the chemicals in the match head are consumed and transformed into different substances.
-
Color Change: The wood turns black (charred) as a result of the chemical breakdown of cellulose.
-
Formation of Ash: The remaining residue is ash, a different substance composed of the inorganic materials left behind after combustion.
Deeper Dive into the Chemical Equations
While the complete chemical equations for the burning of a match are complex and involve multiple reactions, we can represent simplified versions:
-
Reaction of red phosphorus and potassium chlorate: This initial reaction is difficult to represent with a single, balanced equation due to the multiple intermediate products. The overall effect, however, is the release of oxygen and heat.
-
Combustion of sulfur: S(s) + O₂(g) → SO₂(g)
-
Combustion of cellulose (simplified): Cellulose (C₆H₁₀O₅)ₙ is a complex polymer, and its combustion produces carbon dioxide, water, and heat. A simplified equation is: C₆H₁₀O₅ + 6O₂ → 6CO₂ + 5H₂O
These simplified equations demonstrate the formation of new substances (CO₂, H₂O, SO₂) from the original reactants (sulfur, cellulose, and oxygen), confirming the chemical nature of the change.
Beyond the Match: Combustion in a Broader Context
Understanding the chemical changes involved in burning a match provides a foundation for comprehending combustion processes in general. Combustion is a crucial reaction in various applications, including:
-
Internal Combustion Engines: Powering vehicles and machinery through controlled combustion of fuels like gasoline and diesel.
-
Power Generation: Generating electricity in power plants using fossil fuels or biomass.
-
Heating and Cooking: Providing heat for homes and preparing food using natural gas, propane, or wood.
-
Industrial Processes: Driving various industrial processes through combustion reactions.
Conclusion: The Unassuming Chemical Miracle
Burning a match, a seemingly trivial act, reveals a fascinating world of chemical reactions and transformations. The evidence clearly points to a chemical change, involving the formation of new substances, heat release, gas production, and irreversible alteration of the reactants. This understanding extends far beyond the simple act of lighting a match, offering insight into the fundamental principles of combustion and its far-reaching applications in our daily lives. The next time you strike a match, remember the intricate chemical dance occurring before your eyes, a testament to the power and wonder of chemistry.
Latest Posts
Latest Posts
-
How Does Igneous Rock Become Metamorphic
Apr 01, 2025
-
How Does Friction Affect The Motion Of Objects
Apr 01, 2025
-
Sr Oh 2 Strong Or Weak
Apr 01, 2025
-
A Bond In Which Electrons Are Shared Unequally
Apr 01, 2025
-
Greatest Common Factor Of 32 And 36
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Burning A Match A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
