Sr Oh 2 Strong Or Weak
listenit
Apr 01, 2025 · 5 min read
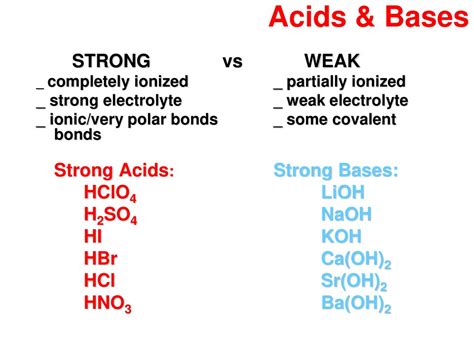
Table of Contents
Sr(OH)₂: Strong or Weak? Understanding its Properties and Applications
The question of whether strontium hydroxide, Sr(OH)₂, is a strong or weak base is a fundamental one in chemistry, impacting its behavior in various applications. While seemingly simple, a thorough understanding requires delving into its properties, dissociation in water, and its practical implications. This comprehensive article will explore these aspects in detail, providing a complete picture of Sr(OH)₂'s strength and its role in different contexts.
Defining Strong and Weak Bases
Before examining Sr(OH)₂, let's clarify the distinction between strong and weak bases. A strong base is a base that completely dissociates into its ions in an aqueous solution. This means that when dissolved in water, it essentially breaks down entirely into its constituent cation (in this case, Sr²⁺) and hydroxide anions (OH⁻). Conversely, a weak base only partially dissociates, meaning a significant portion of the base remains in its molecular form, and only a small amount dissociates into ions. This difference significantly influences the pH and reactivity of the solution.
Sr(OH)₂: A Strong Base
Strontium hydroxide, Sr(OH)₂, is classified as a strong base. This is because it readily and almost completely dissociates in water, according to the following equation:
Sr(OH)₂(s) → Sr²⁺(aq) + 2OH⁻(aq)
The equilibrium lies heavily to the right, indicating a high degree of dissociation. This complete dissociation results in a high concentration of hydroxide ions (OH⁻) in solution, leading to a highly alkaline pH.
Factors Affecting Dissociation
While Sr(OH)₂ is considered a strong base, several factors can subtly influence the extent of its dissociation:
-
Concentration: At very high concentrations, even strong bases might exhibit slightly less than complete dissociation due to ion pairing. However, at typical concentrations used in most experiments and applications, the effect is negligible.
-
Temperature: Temperature changes can impact the solubility and consequently the effective concentration of Sr(OH)₂, thus slightly altering the degree of dissociation. Generally, increased temperature increases solubility and therefore the concentration of hydroxide ions.
-
Solvent: The solvent used can play a role. While water is the typical solvent, using a different solvent could change the dielectric constant and thus affect the interaction between the ions, potentially influencing the extent of dissociation.
Comparing Sr(OH)₂ to Other Bases
Understanding the strength of Sr(OH)₂ requires comparing it to other bases, both strong and weak.
Strong Bases: Sr(OH)₂ shares similarities with other Group 2 hydroxides like Ca(OH)₂ (calcium hydroxide) and Ba(OH)₂ (barium hydroxide), which are also strong bases. The trend of increasing basicity within Group 2 hydroxides reflects the increasing size and decreasing charge density of the metal cations, which influences the stability of the hydroxide lattice. However, the solubility of these hydroxides varies, affecting their practical use. Sr(OH)₂'s solubility is relatively higher than Ca(OH)₂ but lower than Ba(OH)₂.
Weak Bases: In contrast to Sr(OH)₂, weak bases like ammonia (NH₃) and many organic amines only partially dissociate. This results in a much lower concentration of hydroxide ions in solution and a less alkaline pH compared to solutions of Sr(OH)₂ at similar concentrations.
Applications of Strontium Hydroxide
The strong base nature of Sr(OH)₂ makes it useful in several applications:
-
Sugar Refining: Sr(OH)₂ has been traditionally used in the refining of beet sugar. Its strong basicity aids in the precipitation of impurities, leading to a purer sugar product. However, environmental concerns related to strontium's presence have led to a reduction in its use in some regions.
-
Chemical Synthesis: As a strong base, Sr(OH)₂ can participate in various chemical reactions as a reactant or catalyst. Its role often involves neutralization reactions or the generation of hydroxide ions needed for specific chemical transformations.
-
Lubricant Additives: In some lubricant formulations, Sr(OH)₂ might be included to improve certain properties of the lubricant, although its use in this area may be limited and dependent on specific applications.
-
Water Treatment (limited): Although not as widespread as other bases, Sr(OH)₂ has been explored in niche water treatment applications, potentially involving neutralization or precipitation of certain contaminants. However, its use is less common compared to other alkaline earth metal hydroxides due to cost and environmental considerations.
Safety Considerations
It's crucial to handle Sr(OH)₂ with appropriate safety precautions. As a strong base, it is corrosive and can cause skin and eye irritation. Always wear protective gear, including gloves and eye protection, when handling Sr(OH)₂. Proper ventilation should be ensured to avoid inhalation of dust.
Advanced Concepts and Research
The study of Sr(OH)₂ extends beyond its simple classification as a strong base. Research into its properties and applications often involves more advanced concepts:
-
Solubility and Thermodynamics: A deeper understanding of Sr(OH)₂'s solubility in different solvents and at various temperatures requires analyzing its thermodynamic properties, including enthalpy and entropy changes during dissolution.
-
Crystal Structure and Lattice Energy: The crystal structure and lattice energy of Sr(OH)₂ affect its solubility and reactivity. Research in this area involves techniques like X-ray diffraction to determine the crystal structure and computational methods to calculate lattice energies.
-
Reaction Kinetics: Studying the reaction kinetics of Sr(OH)₂ in various chemical processes helps optimize reaction conditions and improve yields.
-
Environmental Impact: The environmental impact of strontium and its compounds, including Sr(OH)₂, is an important area of research, considering potential risks to ecosystems and human health.
Conclusion
Sr(OH)₂ is definitively a strong base, exhibiting almost complete dissociation in aqueous solutions. Its strong basicity leads to a high concentration of hydroxide ions, influencing its pH and reactivity. Although commonly considered a strong base, factors such as concentration and temperature can subtly affect its dissociation. Its applications, while historically significant in sugar refining and niche chemical processes, are now becoming more specialized, often influenced by cost and environmental concerns. Ongoing research continues to explore its properties and its potential roles in various fields, considering not only its chemical behavior but also its environmental impact and safety considerations. Understanding Sr(OH)₂'s strength and its implications is crucial for various chemical and industrial applications, emphasizing the need for careful handling and responsible utilization.
Latest Posts
Latest Posts
-
Irrational Numbers Between 1 And 6
Apr 02, 2025
-
In A Hypertonic Solution A Cell Will
Apr 02, 2025
-
How Many Kilograms Are In 5000 Grams
Apr 02, 2025
-
Solve This Inequality 3b 7 32
Apr 02, 2025
-
Number Of Valence Electrons In Ar
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Sr Oh 2 Strong Or Weak . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
