A Bond In Which Electrons Are Shared Unequally
listenit
Mar 20, 2025 · 6 min read
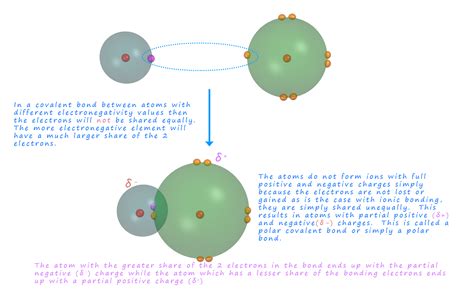
Table of Contents
A Bond in Which Electrons are Shared Unequally: Understanding Polar Covalent Bonds
A fundamental concept in chemistry is the covalent bond, where atoms share electrons to achieve a more stable electron configuration. However, not all covalent bonds are created equal. While some involve an equal sharing of electrons, others exhibit an unequal distribution, leading to a polar covalent bond. Understanding this difference is crucial for comprehending the properties of molecules and their interactions. This article delves deep into the nature of polar covalent bonds, exploring their formation, characteristics, and significance in various chemical and biological systems.
What is a Polar Covalent Bond?
A polar covalent bond is a type of chemical bond where electrons are shared unequally between two atoms. This unequal sharing arises from a difference in the electronegativity of the atoms involved. Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond. Atoms with higher electronegativity exert a stronger pull on the shared electrons, resulting in a partial negative charge (δ-) on the more electronegative atom and a partial positive charge (δ+) on the less electronegative atom.
This difference in charge creates a dipole moment, a measure of the polarity of the bond. The dipole moment is a vector quantity, having both magnitude (the size of the charge separation) and direction (pointing from the positive to the negative pole). The larger the electronegativity difference between the atoms, the greater the dipole moment and the more polar the bond.
Contrasting with Nonpolar Covalent Bonds
In contrast to polar covalent bonds, nonpolar covalent bonds involve the equal sharing of electrons between two atoms. This typically occurs when the atoms have similar or identical electronegativities, such as in a diatomic molecule like O₂ or Cl₂. In these cases, the electron density is evenly distributed, and no significant dipole moment is observed.
Factors Influencing Polarity
Several factors contribute to the polarity of a covalent bond:
1. Electronegativity Difference
The most significant factor determining the polarity of a bond is the difference in electronegativity between the two atoms involved. The greater the difference, the more polar the bond. Electronegativity values are typically represented on the Pauling scale, with fluorine (F) having the highest electronegativity (4.0).
- Large Electronegativity Difference: Leads to highly polar bonds, often exhibiting ionic character.
- Small Electronegativity Difference: Leads to slightly polar bonds, with a minimal charge separation.
- Zero Electronegativity Difference: Results in a nonpolar covalent bond.
2. Bond Length
The distance between the two atoms also influences polarity. Shorter bond lengths generally lead to stronger interactions and increased polarity, as the electrons are held more tightly.
3. Molecular Geometry
The overall shape of the molecule plays a crucial role in determining the molecule's net dipole moment. Even if individual bonds are polar, the molecule as a whole may be nonpolar if the bond dipoles cancel each other out due to symmetry. For example, carbon dioxide (CO₂) has two polar C=O bonds, but the linear geometry causes the bond dipoles to cancel, resulting in a nonpolar molecule. Water (H₂O), on the other hand, has a bent geometry, resulting in a net dipole moment despite the relatively smaller electronegativity difference between oxygen and hydrogen.
Identifying Polar Covalent Bonds
Determining whether a bond is polar can be done by considering the electronegativity values of the atoms involved. While a precise numerical cutoff isn't universally agreed upon, a difference of around 0.5 to 1.7 on the Pauling scale generally indicates a polar covalent bond. Differences greater than 1.7 often suggest an ionic bond, where electrons are essentially transferred rather than shared.
Properties of Molecules with Polar Covalent Bonds
Molecules containing polar covalent bonds exhibit several unique properties:
1. Higher Boiling and Melting Points
Polar molecules tend to have higher boiling and melting points than nonpolar molecules of similar size and mass. This is due to the stronger intermolecular forces between polar molecules, such as dipole-dipole interactions and hydrogen bonding. These forces require more energy to overcome, leading to higher boiling and melting points.
2. Solubility in Polar Solvents
Polar molecules are generally soluble in polar solvents, such as water, because of the strong interactions between the dipoles. "Like dissolves like" is a common rule of thumb in solubility. Nonpolar molecules, however, are more soluble in nonpolar solvents.
3. Higher Dielectric Constants
Polar molecules have higher dielectric constants compared to nonpolar molecules. This means they can reduce the electrostatic forces between charged particles, making them excellent solvents for ionic compounds.
4. Reactivity
Polar covalent bonds can be more reactive than nonpolar bonds. The partial charges on the atoms involved create regions of higher and lower electron density, making them susceptible to nucleophilic and electrophilic attacks.
Examples of Polar Covalent Bonds
Numerous molecules in nature and synthetic chemistry contain polar covalent bonds. Some notable examples include:
- Water (H₂O): The oxygen atom is significantly more electronegative than the hydrogen atoms, leading to highly polar O-H bonds and a substantial net dipole moment.
- Ammonia (NH₃): Nitrogen is more electronegative than hydrogen, resulting in polar N-H bonds and a polar molecule overall.
- Hydrogen Fluoride (HF): Fluorine's high electronegativity creates a highly polar H-F bond.
- Carbonyl Group (C=O): The oxygen atom is more electronegative than the carbon atom, leading to a polar C=O bond, a functional group found in many organic molecules such as ketones, aldehydes and carboxylic acids.
- Hydroxyl Group (-OH): A polar functional group found in alcohols and carboxylic acids. The oxygen atom is highly electronegative.
Importance in Biology and Chemistry
Polar covalent bonds are essential for life and numerous chemical processes. The polarity of water, for instance, is crucial for its role as a solvent and its involvement in various biological reactions. The polar nature of many biomolecules, including proteins and nucleic acids, determines their structure and function. Polar covalent bonds also play a significant role in various chemical reactions, influencing reactivity and selectivity. Furthermore, many important chemical processes in industrial settings rely on the properties of compounds containing polar covalent bonds.
Conclusion
Polar covalent bonds are a fundamental aspect of chemistry and biology. The unequal sharing of electrons creates a dipole moment, influencing the physical and chemical properties of molecules. Understanding the factors that determine the polarity of a bond and the resulting properties of polar molecules is critical for comprehending a wide range of chemical and biological phenomena. From the life-sustaining properties of water to the intricacies of enzyme-substrate interactions, the concept of the polar covalent bond is central to our understanding of the natural world and the design of new materials and technologies. Further research continues to expand our understanding of the nuances of polar covalent bonding, especially as it relates to complex molecules and sophisticated chemical processes. The ability to predict and control the polarity of bonds has far-reaching implications in materials science, drug design and many other fields.
Latest Posts
Latest Posts
-
How Many Feet Is 15 Miles
Mar 20, 2025
-
The Phosphorus Cycle Differs From The Biogeochemical Cycles In That
Mar 20, 2025
-
How Many Neutrons Are In Nitrogen
Mar 20, 2025
-
What Is 30 Percent Of 9
Mar 20, 2025
-
How Many Quarts In 6 Pints
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about A Bond In Which Electrons Are Shared Unequally . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
