What Is The Density Of Rubbing Alcohol In Grams
listenit
Mar 31, 2025 · 5 min read
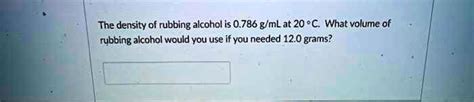
Table of Contents
What is the Density of Rubbing Alcohol in Grams? A Comprehensive Guide
Rubbing alcohol, also known as isopropyl alcohol (IPA), is a common household item with numerous uses, from cleaning surfaces to disinfecting wounds. Understanding its density is crucial for various applications, from precise dilutions in scientific experiments to ensuring accurate measurements in industrial processes. This comprehensive guide will delve into the density of rubbing alcohol in grams, exploring the factors that influence it and providing practical applications of this knowledge.
Understanding Density:
Before we dive into the specifics of rubbing alcohol density, let's establish a clear understanding of what density means. Density is defined as the mass of a substance per unit volume. It's typically expressed in grams per milliliter (g/mL) or grams per cubic centimeter (g/cm³), with both units being equivalent. The formula for density is:
Density = Mass / Volume
This means that a higher density indicates a greater mass packed into a given volume. For instance, a substance with a higher density than water will sink in water, while a substance with a lower density will float.
Factors Affecting the Density of Rubbing Alcohol:
The density of rubbing alcohol isn't a fixed constant. Several factors can influence its value:
1. Concentration of Isopropyl Alcohol:
The most significant factor affecting rubbing alcohol density is its concentration. Rubbing alcohol isn't pure isopropyl alcohol; it's typically a solution of IPA and water. The percentage of IPA in the solution directly impacts the density. Higher concentrations of IPA generally lead to a higher density because IPA has a higher density than water. Common concentrations are 70%, 90%, and 99%, each exhibiting a slightly different density.
2. Temperature:
Temperature plays a crucial role. Like most substances, the density of rubbing alcohol decreases as temperature increases. This is because the molecules gain kinetic energy and spread out, occupying a larger volume. Conversely, a decrease in temperature leads to a denser substance as molecules move closer together. Therefore, it's vital to specify the temperature when reporting the density of rubbing alcohol.
3. Presence of Additives:
Some rubbing alcohol formulations contain additives like denaturants (to make it unsuitable for consumption) or other chemicals to enhance its properties. These additives can slightly alter the overall density compared to pure IPA or standard solutions. The nature and concentration of these additives would need to be considered for precise density calculations.
Typical Density Values of Rubbing Alcohol:
While precise values can vary depending on the concentration, temperature, and any additives present, we can provide typical ranges:
-
70% Isopropyl Alcohol: The most common concentration, often found in drugstores, typically has a density ranging from approximately 0.87 g/mL to 0.89 g/mL at room temperature (around 20-25°C).
-
90% Isopropyl Alcohol: A higher concentration of IPA, this solution will have a higher density, generally falling in the range of 0.89 g/mL to 0.91 g/mL at room temperature.
-
99% Isopropyl Alcohol: Almost pure isopropyl alcohol, this concentration has the highest density, typically ranging from approximately 0.91 g/mL to 0.92 g/mL at room temperature.
It's crucial to remember that these are approximate values. The actual density will depend on the specific product and the conditions under which the measurement is taken. Always refer to the product's specification sheet for the most accurate density information.
Practical Applications of Knowing the Density of Rubbing Alcohol:
Understanding the density of rubbing alcohol has several practical applications:
1. Accurate Dilutions:
In scientific experiments or industrial settings, accurate dilutions are crucial. Knowing the density allows precise calculations to achieve the desired concentration. For instance, if you need to prepare a specific volume of a diluted solution from a stock solution of known density, density values are essential for correct calculations.
2. Quality Control:
In manufacturing and quality control processes, measuring the density of rubbing alcohol can help verify the concentration and purity of the product. Discrepancies from the expected density can indicate potential contamination or errors in the manufacturing process.
3. Determining Volume from Mass:
Using the density formula, if you know the mass of rubbing alcohol, you can calculate its volume. This is useful in situations where measuring volume directly might be difficult. For instance, if you have a certain weight of rubbing alcohol, you can calculate the corresponding volume.
4. Mixing Solutions:
When mixing rubbing alcohol with other liquids, understanding density helps predict the behavior of the mixture. Knowing the relative densities of the components can aid in determining the final density and the characteristics of the mixture.
5. Specific Gravity Calculations:
Specific gravity is the ratio of the density of a substance to the density of a reference substance (typically water). Knowing the density of rubbing alcohol allows easy calculation of its specific gravity, which is often used in various industrial and scientific contexts.
Measuring the Density of Rubbing Alcohol:
While you might not need to measure the density in everyday use, knowing the methods is useful for verification or for more specialized applications. The most common method involves using a precise measuring device:
-
Using a Pycnometer: A pycnometer is a specialized glass instrument designed for precise density measurements. It involves carefully weighing the pycnometer empty, filled with water, and then filled with the rubbing alcohol sample. The density is calculated using the mass difference and known volume of the pycnometer.
-
Using a Hydrometer: A hydrometer is a simple instrument that floats in a liquid, and its depth of immersion indicates the density of the liquid. Hydrometers are calibrated for specific substances and temperature ranges, making them convenient for quick density estimations.
-
Using a Density Meter: Digital density meters provide highly precise and automated density measurements. These instruments are commonly used in laboratories and industrial settings requiring accurate density data.
Conclusion:
The density of rubbing alcohol is not a single fixed value; it varies depending on factors such as the concentration of isopropyl alcohol, temperature, and any additives present. While approximate ranges are provided, precise values should always be obtained from the product's specification sheet. Understanding the density of rubbing alcohol is vital for numerous applications, from accurate dilutions in scientific experiments to quality control in industrial processes. Knowing how to measure density and its practical applications allows for greater precision and accuracy in many situations requiring the use of isopropyl alcohol. Remember to always handle rubbing alcohol responsibly and follow safety precautions.
Latest Posts
Latest Posts
-
Lewis Acid Vs Bronsted Lowry Acid
Apr 01, 2025
-
What Is Square Root Of 63
Apr 01, 2025
-
Least Common Multiple 2 And 4
Apr 01, 2025
-
How Does Igneous Rock Become Metamorphic
Apr 01, 2025
-
How Does Friction Affect The Motion Of Objects
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Density Of Rubbing Alcohol In Grams . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
