What Is The Difference Between A Solution And A Suspension
listenit
Mar 31, 2025 · 6 min read
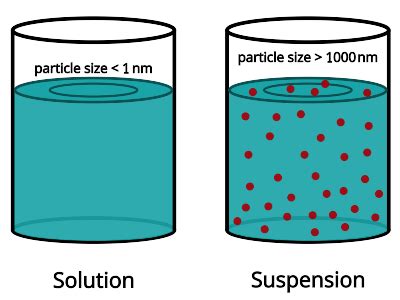
Table of Contents
- What Is The Difference Between A Solution And A Suspension
- Table of Contents
- What's the Difference Between a Solution and a Suspension? A Deep Dive into Mixture Chemistry
- Particle Size: The Defining Difference
- Solutions: Homogeneous at the Molecular Level
- Suspensions: Heterogeneous with Larger Particles
- Settling and Stability: A Test of Time
- Solutions: Lasting Stability
- Suspensions: Settling and Instability
- Filtration: Separating the Components
- Solutions: Passing Through Filters Unchanged
- Suspensions: Easily Separated by Filtration
- The Tyndall Effect: Light Scattering and Visibility
- Solutions: No Tyndall Effect
- Suspensions: Strong Tyndall Effect
- Real-World Applications: Solutions and Suspensions in Action
- Solutions: Ubiquitous in Everyday Life and Industry
- Suspensions: Specific Uses Leveraging their Properties
- Colloids: Bridging the Gap Between Solutions and Suspensions
- Conclusion: Understanding the Distinctions for Practical Applications
- Latest Posts
- Latest Posts
- Related Post
What's the Difference Between a Solution and a Suspension? A Deep Dive into Mixture Chemistry
Understanding the difference between a solution and a suspension is fundamental to chemistry and numerous applications in everyday life. While both are mixtures, their distinct properties and behaviors stem from the size and interaction of their components. This article delves deep into the characteristics of solutions and suspensions, exploring their differences through various aspects, including particle size, settling, filtration, and Tyndall effect. We will also examine their real-world applications and implications.
Particle Size: The Defining Difference
The most crucial difference between a solution and a suspension lies in the size of the particles comprising the mixture. This seemingly simple distinction dictates a vast array of properties and behaviors.
Solutions: Homogeneous at the Molecular Level
In a solution, the solute (the substance being dissolved) is broken down into individual molecules or ions, which are dispersed uniformly throughout the solvent (the substance doing the dissolving). These particles are incredibly small, typically less than 1 nanometer (nm) in diameter. This microscopic uniformity is why solutions appear homogenous; meaning, they have a uniform composition throughout. Think of salt dissolved in water – you can't see the individual salt particles, only the clear water.
Suspensions: Heterogeneous with Larger Particles
Suspensions, on the other hand, are characterized by relatively large solute particles, usually greater than 1000 nm (1 micrometer or 1µm) in diameter. These particles are readily visible to the naked eye or under a basic microscope. Because these particles are so large, they remain essentially unchanged and are not uniformly dispersed within the solvent; they are distributed throughout the mixture but can settle over time. This non-uniformity results in a heterogeneous mixture – meaning, its composition is not uniform. Think of sand in water – the sand particles are easily visible, and they settle to the bottom if the mixture is left undisturbed.
Settling and Stability: A Test of Time
The difference in particle size directly impacts the stability of solutions and suspensions over time.
Solutions: Lasting Stability
Solutions exhibit exceptional stability. Because the solute particles are so small and uniformly dispersed, they don't settle out, even over extended periods. The attractive forces between solute and solvent molecules are strong enough to keep them consistently mixed. A properly prepared saltwater solution will remain a homogenous mixture indefinitely.
Suspensions: Settling and Instability
Suspensions are inherently unstable. The larger solute particles in a suspension are susceptible to gravity, leading to sedimentation – the particles settling to the bottom of the container. This settling is often quite rapid, depending on the particle size and density, and the viscosity of the solvent. Shaking or stirring a suspension can temporarily redistribute the particles, but they will eventually settle again.
Filtration: Separating the Components
Filtration, a process of separating solids from liquids or gases, provides another way to differentiate solutions and suspensions.
Solutions: Passing Through Filters Unchanged
Solutions pass through ordinary filter paper without any separation of their components. The solute particles are too small to be trapped by the filter pores. The filtrate (the liquid that passes through the filter) will have the same composition as the original solution.
Suspensions: Easily Separated by Filtration
Suspensions can be easily separated by filtration. The larger solute particles are readily trapped by the filter paper, leaving a clear filtrate of the solvent. This simple technique demonstrates the heterogeneous nature of suspensions, where the components can be physically separated.
The Tyndall Effect: Light Scattering and Visibility
The Tyndall effect, the scattering of light by colloidal particles, provides a visual distinction between solutions and suspensions (and colloids, which fall between solutions and suspensions).
Solutions: No Tyndall Effect
Solutions do not exhibit the Tyndall effect. The solute particles are too small to scatter visible light significantly; light passes straight through the solution without any noticeable scattering.
Suspensions: Strong Tyndall Effect
Suspensions display a pronounced Tyndall effect. The larger particles scatter light effectively, resulting in a visible beam of light when a light source is shone through the suspension. You can observe this effect easily by shining a flashlight through a glass of muddy water – the light beam will be clearly visible due to the scattering of light by the suspended mud particles. This light scattering property is a key indicator of a heterogeneous mixture.
Real-World Applications: Solutions and Suspensions in Action
Solutions and suspensions have numerous applications across various fields, showcasing their unique properties.
Solutions: Ubiquitous in Everyday Life and Industry
Solutions are ubiquitous in our everyday lives and essential in many industrial processes. Examples include:
- Saltwater: A simple solution crucial for various applications, from cooking to desalination.
- Sugar in water: A common solution used in beverages and food preparation.
- Pharmaceutical solutions: Many medications are administered as solutions for easy absorption and consistent dosage.
- Cleaning solutions: Many household cleaners are solutions designed to dissolve dirt and grime.
- Electrolyte solutions: crucial for batteries and electrochemical processes.
Suspensions: Specific Uses Leveraging their Properties
Suspensions find niche applications where their unique properties are advantageous:
- Paints: Pigments are suspended in a liquid medium, allowing for even coating and controlled application.
- Muddy water: A natural suspension illustrating the principles of sedimentation and filtration.
- Some medications: Some oral medications utilize suspensions to deliver drugs in a palatable or easily absorbed form.
- Antacids: Many antacids are suspensions of insoluble solids in liquid, acting to neutralize stomach acid.
- Cosmetics: Certain creams and lotions are suspensions of solids in a base, offering controlled release and texture.
Colloids: Bridging the Gap Between Solutions and Suspensions
While solutions and suspensions represent distinct categories, it's crucial to acknowledge colloids. Colloids are mixtures whose particle size falls between that of solutions and suspensions, typically ranging from 1 to 1000 nm. Colloidal particles are too large to be truly dissolved but too small to settle out readily. Milk, fog, and gelatin are common examples of colloids. They exhibit the Tyndall effect but do not readily settle like suspensions. This illustrates the spectrum of mixtures and the gradual transition in properties based on particle size.
Conclusion: Understanding the Distinctions for Practical Applications
The differences between solutions and suspensions are significant, dictating their behavior, stability, and applications. The particle size is the key differentiator, determining whether the mixture appears homogeneous or heterogeneous, its stability over time, its response to filtration, and its light scattering properties. Understanding these differences is crucial in various scientific and industrial contexts, enabling the selection and utilization of the appropriate mixture type for specific purposes. Whether you're working with a simple saltwater solution or a complex pharmaceutical suspension, understanding these fundamental principles will enhance your comprehension of chemistry and its practical applications.
Latest Posts
Latest Posts
-
Atoms Have No Electric Charge Because They Have
Apr 02, 2025
-
How Many Electrons Can Fit In The 3rd Energy Level
Apr 02, 2025
-
What Is 3 5 1 3
Apr 02, 2025
-
18 Is What Percent Of 27
Apr 02, 2025
-
Explain The Relationship Between Monomers And Polymers
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between A Solution And A Suspension . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
