What Is A Characteristic Of A Base
listenit
Mar 31, 2025 · 6 min read
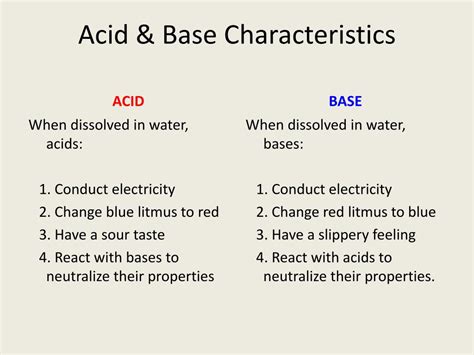
Table of Contents
What is a Characteristic of a Base? Understanding the Properties of Bases
Bases are fundamental chemical compounds with unique properties that distinguish them from acids. Understanding these characteristics is crucial in various fields, from chemistry and biology to environmental science and everyday life. This comprehensive article delves into the defining characteristics of bases, exploring their chemical behavior, physical properties, and practical applications. We'll explore different types of bases, their reactions, and the importance of understanding their properties.
Defining a Base: More Than Just a Bitter Taste
While the traditional understanding of bases might involve a bitter taste and slippery feel (like soap), a more precise definition centers on their chemical behavior. A base, also known as an alkali (though not all bases are alkalis), is a substance that can:
-
Accept a proton (H⁺): This is the most fundamental characteristic according to the Brønsted-Lowry theory. A base readily accepts a hydrogen ion (proton) from an acid. This acceptance is a key feature of acid-base neutralization reactions.
-
Donate a hydroxide ion (OH⁻): According to the Arrhenius theory, a base is a substance that dissociates in water to produce hydroxide ions. These hydroxide ions are responsible for many of the characteristic properties of bases.
-
Increase the hydroxide ion concentration in a solution: This increase in OH⁻ concentration leads to a higher pH, making the solution alkaline.
Key Characteristics of Bases: A Deeper Dive
Let's explore the key characteristics of bases in more detail:
1. pH Value: Above 7
The pH scale, ranging from 0 to 14, measures the acidity or alkalinity of a solution. A pH of 7 is neutral. Bases have a pH greater than 7. The higher the pH, the stronger the base. For example, a pH of 10 is more alkaline than a pH of 8. This pH value is a direct consequence of the increased hydroxide ion concentration in a base solution.
2. Reaction with Acids: Neutralization
A hallmark of bases is their reaction with acids in a process called neutralization. When a base reacts with an acid, they neutralize each other, producing salt and water. This reaction is exothermic, meaning it releases heat.
Example: The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl, table salt) and water (H₂O):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This neutralization reaction is crucial in various applications, such as titrations (used to determine the concentration of an unknown solution) and controlling pH in industrial processes.
3. Taste and Feel: Bitter and Slippery
While not a definitive chemical characteristic, many bases exhibit a bitter taste and a slippery or soapy feel. These sensations are linked to the interaction of hydroxide ions with the receptors on your tongue and skin. However, it is crucial to avoid tasting or touching unknown substances, as many bases can be corrosive and harmful.
4. Electrical Conductivity: Varies with Strength
Strong bases, like sodium hydroxide (NaOH) and potassium hydroxide (KOH), readily dissociate into ions in water, resulting in high electrical conductivity. Weak bases, on the other hand, only partially dissociate, leading to lower conductivity. The presence of freely moving ions in solution allows the base solution to conduct electricity.
5. Chemical Reactions: Beyond Neutralization
Bases participate in several other chemical reactions besides neutralization. They can:
- React with certain metals: Some bases, particularly molten bases, can react with certain metals, like aluminum, producing hydrogen gas.
- Undergo hydrolysis: Certain bases can react with water to form hydroxide ions and their conjugate acid. This reaction is particularly relevant for weak bases.
- React with esters and amides: Bases can participate in saponification, a process that involves the hydrolysis of esters, typically used in soap making. They can also react with amides in various organic reactions.
Types of Bases: A Diverse Family
Bases come in various forms, categorized based on their strength and chemical structure:
1. Strong Bases: Complete Dissociation
Strong bases completely dissociate in water, releasing a high concentration of hydroxide ions. Examples include:
- Sodium hydroxide (NaOH): Commonly known as lye or caustic soda, used in soap making, drain cleaners, and various industrial processes.
- Potassium hydroxide (KOH): Similar to NaOH, used in various industrial applications.
- Calcium hydroxide (Ca(OH)₂): Also known as slaked lime, used in construction and water treatment.
2. Weak Bases: Partial Dissociation
Weak bases only partially dissociate in water, resulting in a lower concentration of hydroxide ions. Examples include:
- Ammonia (NH₃): A common household cleaner and component of fertilizers.
- Sodium carbonate (Na₂CO₃): Washing soda, used in detergents and cleaning products.
- Many organic amines: These contain nitrogen atoms and are found in many biological molecules and pharmaceuticals.
3. Lewis Bases: Electron Pair Donors
Beyond Brønsted-Lowry bases, the Lewis theory expands the definition of a base. A Lewis base is a substance that can donate a pair of electrons to form a coordinate covalent bond. Many substances that aren't Brønsted-Lowry bases can act as Lewis bases.
Example: Ammonia (NH₃) can act as a Lewis base because the nitrogen atom has a lone pair of electrons that can be donated to an electron-deficient species (a Lewis acid).
Applications of Bases: A Wide Range of Uses
The properties of bases make them indispensable in various applications:
1. Industrial Applications:
- Soap making (saponification): Bases are essential for converting fats and oils into soap.
- Paper production: Bases help in the pulping process, separating fibers from wood.
- Textile industry: Bases are used in dyeing and processing fabrics.
- Water treatment: Bases are used to adjust the pH of water and to remove acidity.
- Fertilizer production: Ammonia, a weak base, is a crucial component of many fertilizers.
2. Everyday Applications:
- Cleaning products: Many household cleaners, such as drain cleaners and oven cleaners, contain strong bases.
- Baking: Baking soda (sodium bicarbonate) is a weak base used as a leavening agent.
- Antacids: Some antacids contain weak bases to neutralize stomach acid.
- Agriculture: Lime (calcium carbonate), a weak base, is often used to adjust soil pH.
3. Biological Applications:
- Maintaining pH balance in the body: The human body utilizes buffers, which involve weak acids and bases to maintain a stable pH.
- Enzyme function: The activity of many enzymes depends on the pH of their environment, which is regulated by acids and bases.
- DNA structure: The structure and function of DNA are influenced by pH.
Safety Precautions: Handling Bases with Care
Many bases are corrosive and can cause serious harm if not handled carefully. Always wear appropriate protective gear, such as gloves, eye protection, and lab coats, when working with bases. In case of contact with skin or eyes, immediately flush the affected area with plenty of water and seek medical attention if necessary. Proper ventilation is also important when working with bases, as some can release harmful vapors.
Conclusion: Understanding the Importance of Bases
Bases are crucial components of the chemical world, playing essential roles in numerous industrial, everyday, and biological processes. Their defining characteristics, such as their ability to accept protons, increase hydroxide ion concentration, and react with acids, distinguish them from acids. Understanding these characteristics, along with the various types of bases and their diverse applications, is fundamental to a comprehensive understanding of chemistry and its applications in various fields. Always remember to handle bases with the necessary care and safety precautions to avoid potential hazards.
Latest Posts
Latest Posts
-
How Do You Find The Slope Of A Line Perpendicular
Apr 02, 2025
-
Divide 2x2 17x 35 By X 5
Apr 02, 2025
-
Number Of Valence Electrons For Carbon
Apr 02, 2025
-
How To Find Density Of A Solution
Apr 02, 2025
-
What Is The Gcf Of 45 And 75
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is A Characteristic Of A Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
