What Happens To Atoms During A Chemical Reaction
listenit
Mar 21, 2025 · 6 min read
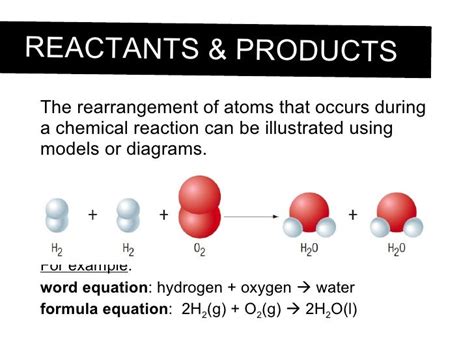
Table of Contents
What Happens to Atoms During a Chemical Reaction?
Chemical reactions are the fundamental processes that govern the changes we observe in the world around us. From the rusting of iron to the digestion of food, these reactions involve the rearrangement of atoms, the fundamental building blocks of matter. Understanding what happens to atoms during a chemical reaction is key to grasping the nature of chemistry itself. This article delves deep into the atomic processes involved, exploring concepts like electron transfer, bond breaking and formation, and the conservation of atoms.
The Indivisible Building Blocks: Atoms and Their Structure
Before diving into the intricacies of chemical reactions, let's revisit the basic structure of an atom. An atom consists of a dense central nucleus containing positively charged protons and electrically neutral neutrons. Surrounding this nucleus is a cloud of negatively charged electrons, orbiting in specific energy levels or shells. The number of protons in an atom's nucleus defines its atomic number and determines its identity as a particular element (e.g., hydrogen, oxygen, carbon). The electrons, particularly those in the outermost shell (valence electrons), are the key players in chemical reactions.
Valence Electrons: The Driving Force Behind Reactions
Valence electrons are responsible for the chemical behavior of an atom. Atoms strive to achieve a stable electron configuration, often by filling their outermost electron shell. This stable configuration is typically achieved by having eight electrons in the valence shell (the octet rule), although there are exceptions, particularly for elements in the first and second periods of the periodic table. This drive for stability is the primary driving force behind chemical reactions.
The Dance of Atoms: Bond Breaking and Formation
A chemical reaction fundamentally involves the breaking of existing chemical bonds between atoms and the formation of new bonds to create different molecules. A chemical bond is the attractive force that holds atoms together in a molecule. The principal types of chemical bonds are:
1. Ionic Bonds: The Transfer of Electrons
Ionic bonds form when one atom transfers one or more electrons to another atom. This transfer creates ions: positively charged cations (atoms that have lost electrons) and negatively charged anions (atoms that have gained electrons). The electrostatic attraction between these oppositely charged ions constitutes the ionic bond. A classic example is the formation of sodium chloride (NaCl), common table salt, where sodium (Na) loses an electron to chlorine (Cl).
2. Covalent Bonds: The Sharing of Electrons
Covalent bonds form when atoms share electrons to achieve a stable electron configuration. This sharing creates a strong bond between the atoms, resulting in the formation of molecules. For example, in a water molecule (H₂O), each hydrogen atom shares an electron with the oxygen atom, forming two covalent bonds. Covalent bonds can be polar (unequal sharing of electrons) or nonpolar (equal sharing of electrons), depending on the electronegativity of the atoms involved.
3. Metallic Bonds: A Sea of Electrons
Metallic bonds occur in metals, where valence electrons are delocalized and move freely among the metal atoms. This "sea" of electrons creates a strong attractive force holding the metal atoms together. This unique bonding explains the properties of metals, such as their electrical and thermal conductivity, malleability, and ductility.
The Conservation of Atoms: A Fundamental Principle
A crucial point to remember is that during a chemical reaction, atoms are neither created nor destroyed. This is known as the law of conservation of mass. The atoms simply rearrange themselves, forming new molecules with different properties. The total number of each type of atom remains the same throughout the reaction. This principle is fundamental to balancing chemical equations, which represent the reactants (starting materials) and products (resulting substances) of a reaction.
Examples of Atomic Rearrangement in Chemical Reactions
Let's explore a few specific examples to illustrate the atomic processes involved in chemical reactions:
1. Combustion of Methane (CH₄):
The combustion of methane, a major component of natural gas, involves a reaction with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O).
CH₄ + 2O₂ → CO₂ + 2H₂O
In this reaction:
- The covalent bonds in methane and oxygen molecules are broken.
- The carbon atom in methane forms four new covalent bonds with two oxygen atoms in the carbon dioxide molecule.
- Each hydrogen atom in methane forms a covalent bond with an oxygen atom to form water molecules.
- The number of carbon, hydrogen, and oxygen atoms remains the same before and after the reaction.
2. Rusting of Iron (Fe):
Rusting is an oxidation-reduction reaction where iron reacts with oxygen in the presence of water to form iron(III) oxide (rust).
4Fe + 3O₂ + 6H₂O → 4Fe(OH)₃
This reaction involves:
- The breaking of covalent bonds in oxygen molecules and the formation of new bonds between iron and oxygen atoms.
- The transfer of electrons from iron atoms to oxygen atoms. Iron is oxidized (loses electrons), and oxygen is reduced (gains electrons).
- The formation of hydrated iron(III) oxide, a solid compound.
3. Neutralization Reaction:
A neutralization reaction involves the reaction between an acid and a base to form water and a salt. For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH):
HCl + NaOH → NaCl + H₂O
This reaction includes:
- The breaking of ionic bonds in HCl and NaOH.
- The formation of new ionic bonds in NaCl.
- The formation of covalent bonds in water (H₂O).
- The hydrogen and hydroxide ions combine to form water, which is a neutral molecule.
Factors Influencing Chemical Reactions
Several factors influence the rate and extent of chemical reactions:
- Temperature: Higher temperatures increase the kinetic energy of reactant molecules, leading to more frequent and energetic collisions, which increases the reaction rate.
- Concentration: Higher concentrations of reactants increase the probability of collisions and thus the reaction rate.
- Surface Area: For reactions involving solids, a larger surface area increases the contact between reactants and enhances the reaction rate.
- Catalysts: Catalysts are substances that increase the rate of a reaction without being consumed themselves. They provide an alternative reaction pathway with a lower activation energy.
Conclusion: The Dynamic Nature of Matter
Chemical reactions are dynamic processes that involve the breaking and formation of chemical bonds, resulting in the rearrangement of atoms to form new substances. The conservation of atoms is a fundamental principle governing these reactions, and understanding the behavior of valence electrons is crucial to comprehending the driving forces behind these transformations. By studying these processes, we gain a deeper appreciation for the intricate and ever-changing nature of matter around us. The examples provided only scratch the surface of the vast complexity and diversity of chemical reactions, highlighting the fundamental role of atoms in shaping our world. Further exploration into specific reaction types, reaction mechanisms, and the influence of various factors on reaction rates will deepen your understanding of this fundamental area of chemistry.
Latest Posts
Latest Posts
-
What Is The Percent For 1 20
Mar 21, 2025
-
How Many Lbs Is 114 Kg
Mar 21, 2025
-
Is Freezing A Chemical Or Physical Change
Mar 21, 2025
-
Is Boron Solid Liquid Or Gas
Mar 21, 2025
-
An Atom That Loses An Electron Is Called
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Happens To Atoms During A Chemical Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
