An Atom That Loses An Electron Is Called
listenit
Mar 21, 2025 · 7 min read
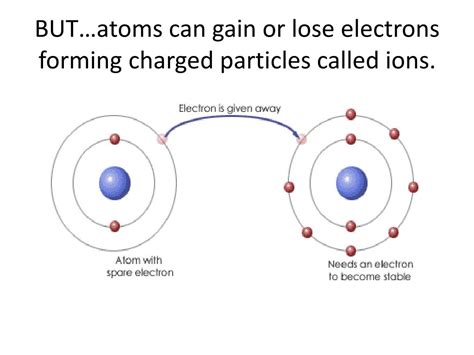
Table of Contents
An Atom That Loses an Electron is Called an Ion: A Deep Dive into Ionization and its Implications
When an atom loses an electron, it's no longer electrically neutral. This fundamental change transforms the atom into something called an ion. Understanding ionization, the process of forming ions, is crucial to grasping a wide range of scientific concepts, from the behavior of gases in neon lights to the intricate mechanisms driving chemical reactions and the very structure of stars. This article delves into the world of ions, explaining what they are, how they form, and their significant role in various scientific fields.
What is an Ion?
An ion is an atom or molecule that has gained or lost one or more electrons, resulting in a net electrical charge. Atoms, in their neutral state, contain an equal number of positively charged protons in their nucleus and negatively charged electrons orbiting around it. This balance ensures that the overall charge is zero. However, when an atom loses an electron, it has more protons than electrons, leading to a net positive charge. This positively charged ion is called a cation. Conversely, when an atom gains an electron, it possesses more electrons than protons, resulting in a net negative charge. This negatively charged ion is known as an anion.
The process of forming an ion is called ionization. This can occur through various mechanisms, including:
Mechanisms of Ionization
-
Electron Transfer: This is perhaps the most common mechanism. When atoms interact, particularly in chemical reactions, electrons can be transferred from one atom to another. The atom that loses electrons becomes a cation, while the atom that gains electrons becomes an anion. This principle underpins the formation of ionic compounds, where oppositely charged ions are held together by electrostatic forces. Think of table salt (NaCl): sodium (Na) loses an electron to become a Na⁺ cation, while chlorine (Cl) gains an electron to become a Cl⁻ anion. The electrostatic attraction between these oppositely charged ions forms the ionic bond.
-
High Temperatures: At extremely high temperatures, such as those found in stars or flames, the kinetic energy of atoms becomes sufficient to overcome the electrostatic attraction between the nucleus and electrons. This leads to the ejection of electrons, creating ions. This process is crucial in plasma physics, where matter exists in a highly ionized state.
-
Radiation: High-energy radiation, like X-rays or gamma rays, can also ionize atoms. The energy from the radiation can directly knock electrons out of their orbitals, leading to the formation of ions. This is a fundamental principle in radiation detection and therapy. Ionizing radiation is highly energetic and can damage biological molecules, making it crucial to understand its effects on living organisms.
-
Electric Fields: Strong electric fields can accelerate charged particles, such as electrons or ions, to high speeds. These high-speed particles can then ionize other atoms through collisions. This principle is used in various technologies, including mass spectrometry and particle accelerators.
Properties of Ions
The properties of ions differ significantly from their neutral counterparts. Several key characteristics are influenced by the gain or loss of electrons:
-
Charge: The most defining characteristic of an ion is its electrical charge. Cations possess a positive charge, while anions possess a negative charge. The magnitude of the charge depends on the number of electrons gained or lost.
-
Size: The size of an ion can differ significantly from the neutral atom. Generally, cations are smaller than their parent atoms because they have lost electrons, resulting in a stronger effective nuclear charge pulling the remaining electrons closer to the nucleus. Conversely, anions are typically larger than their parent atoms because the addition of electrons increases electron-electron repulsion, causing the electron cloud to expand.
-
Chemical Reactivity: Ions are highly reactive due to their electrical charge. They readily participate in chemical reactions to achieve stability, often by forming ionic bonds with oppositely charged ions.
-
Solubility: The solubility of ionic compounds in water and other polar solvents is generally high due to the strong interaction between the charged ions and polar molecules. This is why many ionic compounds dissolve readily in water.
Importance of Ions in Various Fields
The formation and behavior of ions play a crucial role in numerous scientific disciplines:
Chemistry
In chemistry, ions are fundamental building blocks of ionic compounds. Understanding ionic bonding is essential for predicting the properties of these compounds, such as their melting points, boiling points, and solubility. The study of ions and their interactions is critical in areas such as acid-base chemistry, redox reactions, and electrochemistry. Many chemical reactions involve electron transfer, leading to the formation of ions. The concept of oxidation states, which represent the apparent charge of an atom in a compound, is directly related to electron transfer and the formation of ions.
Biology
Ions are essential for many biological processes. For instance, sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and chloride (Cl⁻) ions play vital roles in nerve impulse transmission, muscle contraction, and maintaining osmotic balance in cells. These ions move across cell membranes through ion channels, creating electrical potentials that are crucial for cellular communication and function. Furthermore, many enzymes require metal ions as cofactors for their catalytic activity. Trace amounts of various metal ions are crucial for maintaining health and normal bodily functions.
Physics
In physics, ions are crucial in fields like plasma physics, where matter exists in a highly ionized state. Plasmas are found in stars, fluorescent lights, and fusion reactors. The behavior of ions in electric and magnetic fields is essential for understanding plasma dynamics and applications like plasma etching in semiconductor manufacturing.
Environmental Science
Ions play a significant role in environmental processes. For example, the presence of specific ions in water affects its quality and suitability for drinking and other purposes. The concentration of ions in soil and water influences plant growth and ecosystem health. Understanding ion transport in soil and water is crucial for managing environmental resources and preventing pollution.
Materials Science
Many materials, including ceramics, semiconductors, and alloys, are composed of ions. The properties of these materials, such as their strength, electrical conductivity, and magnetic properties, depend on the types and arrangement of ions in the material's structure.
Applications of Ionization
The principle of ionization finds applications in many technologies and instruments:
-
Mass Spectrometry: Mass spectrometry utilizes ionization to create charged molecules or atoms which can be separated based on their mass-to-charge ratio. This technique is crucial in identifying and quantifying different molecules in a sample, with applications in analytical chemistry, forensics, and proteomics.
-
Flame Photometry and Atomic Absorption Spectroscopy: These analytical techniques utilize the excitation and ionization of atoms in a flame to determine the concentration of specific elements in a sample. These methods have wide applications in environmental monitoring, clinical analysis, and materials characterization.
-
Ionization Detectors: Ionization detectors are used in various instruments, such as gas chromatography and high-performance liquid chromatography (HPLC), to detect and quantify the components separated by these techniques. They rely on the ionization of the separated compounds to create a measurable signal.
-
Radiation Detection: Ionizing radiation detectors, such as Geiger counters, utilize the ionization caused by radiation to detect and measure its intensity. These devices are critical in various applications, including radiation safety monitoring, nuclear medicine, and environmental radiation monitoring.
-
Plasma Technologies: Plasma technologies utilize ionized gases for various industrial applications. Plasma etching and deposition are used in semiconductor manufacturing to create intricate patterns on silicon wafers. Plasma treatment is also utilized for surface modification and sterilization.
Conclusion
In conclusion, understanding the concept of an ion—an atom that has lost or gained electrons—is fundamental to numerous scientific disciplines. The process of ionization, leading to the formation of cations and anions, profoundly affects the chemical, physical, and biological properties of atoms and molecules. Its significance extends across chemistry, biology, physics, environmental science, and materials science, impacting our understanding of the natural world and driving the development of numerous technologies. The myriad applications of ionization highlight its crucial role in modern scientific research and technological advancements. From the intricate workings of biological systems to the vast expanses of the cosmos, the ion's influence is pervasive and profound.
Latest Posts
Latest Posts
-
How Do You Write 3 2 As A Percentage
Mar 22, 2025
-
A Conical Tank With Vertex Down
Mar 22, 2025
-
What Is 60 Percent Of 25
Mar 22, 2025
-
How Do You Write 1 9 As A Decimal
Mar 22, 2025
-
Which Is The Most Reactive Metal
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about An Atom That Loses An Electron Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
