Is Boron Solid Liquid Or Gas
listenit
Mar 21, 2025 · 5 min read
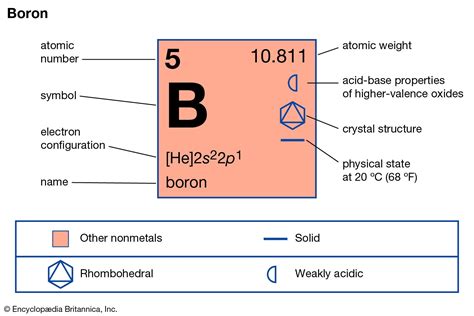
Table of Contents
Is Boron Solid, Liquid, or Gas? A Deep Dive into Boron's Properties
Boron, a fascinating metalloid element, presents unique characteristics that set it apart from typical metals and nonmetals. Understanding its physical state at various temperatures and pressures is crucial to appreciating its applications in diverse fields. This article will delve into the question: Is boron solid, liquid, or gas? We will explore its phase transitions, atomic structure, and the factors influencing its state, providing a comprehensive understanding of this intriguing element.
Boron's Crystalline Structure: The Foundation of its Solid State
At standard temperature and pressure (STP), boron exists solely as a solid. This is primarily due to its robust crystalline structure. Unlike many elements, boron doesn't form a simple cubic or close-packed structure. Instead, it exhibits complex structures with varying degrees of bonding, impacting its physical and chemical properties significantly. The most common crystalline form is α-rhombohedral boron, a complex structure containing 12 icosahedra (clusters of 12 boron atoms) arranged in a unique three-dimensional network.
Icosahedral Units: The Building Blocks of Boron
The icosahedron, a geometric structure with 20 equilateral triangular faces, is fundamental to boron's solid state. These icosahedral units are linked together through additional boron atoms and bonding arrangements, resulting in a highly stable and rigid structure. This intricate bonding network contributes to boron's high melting point (approximately 2076°C) and hardness, making it a challenging material to process.
Variations in Boron's Crystalline Forms
While α-rhombohedral boron is the most common allotrope, other crystalline forms of boron exist, each with slight variations in its atomic arrangement. These differences can influence properties such as density, hardness, and electrical conductivity. These variations arise due to different growth conditions and the presence of impurities. Understanding these variations is vital for tailoring boron's properties for specific applications.
The High Melting Point: Why Boron Remains Solid
The remarkably high melting point of boron is a direct consequence of its strong covalent bonding. Unlike metals which have delocalized electrons creating a "sea" of electrons, boron atoms are strongly bound to each other through covalent bonds. This strong bonding requires a substantial amount of energy to overcome, explaining its resistance to melting at relatively high temperatures. This strong covalent bonding also results in boron's exceptional hardness.
Boron's Transition to Liquid Phase: A High-Temperature Affair
To transition boron from its solid state to a liquid state, extremely high temperatures are required. The precise melting point can vary slightly depending on purity and pressure, but it consistently falls in the region of 2076°C. Above this temperature, the strong covalent bonds begin to break down, allowing boron atoms to move more freely and transition into a liquid phase. However, working with boron at these temperatures presents significant challenges due to its reactivity with many common materials used in high-temperature experiments.
Challenges in Studying Liquid Boron
The extreme temperatures needed to study liquid boron present significant experimental difficulties. Contamination is a major concern, as boron readily reacts with many crucible materials at high temperatures. Accurate measurements of liquid boron's properties, such as viscosity and density, require specialized techniques and robust experimental setups.
The Elusive Gaseous State: Sublimation and Ionization
Unlike many elements that transition directly from solid to liquid and then to gas, boron exhibits a more complex behavior. At extremely high temperatures and under specific conditions, boron can sublime – transitioning directly from solid to gas without passing through the liquid phase. This sublimation process usually occurs at temperatures well above its melting point, and the exact conditions are still under investigation.
Boron's Behavior in Plasma Conditions
In plasma conditions (highly energized gaseous state), boron atoms can become ionized, meaning they lose electrons and become charged particles. This ionization further complicates the study of boron's behavior in the gaseous phase, as the properties of ionized boron differ significantly from neutral boron atoms. Investigating boron's behavior in plasma is crucial in fields like plasma processing and materials science.
Factors Influencing Boron's Phase: Pressure and Impurities
While temperature is the primary factor determining boron's phase transition, pressure and impurities can also play a role. High pressure can potentially alter the equilibrium between boron's solid, liquid, and gaseous states, although this aspect requires further research. Similarly, the presence of impurities can influence boron's melting point and other physical properties, making it crucial to utilize high-purity boron in scientific investigations.
Applications of Boron Across its Phases
Boron's diverse applications are closely linked to its properties in each phase:
Solid Boron: Strength and Functionality
- High-strength materials: Boron's hardness and high melting point make it invaluable in the creation of high-strength materials, particularly in aerospace and defense applications. It's often used as an additive in alloys to enhance their properties.
- Semiconductors: Boron's semi-conducting properties are essential in electronics, particularly in the doping of silicon in semiconductor devices. This precise control over its electrical properties allows for tailored semiconductor behavior.
- Nuclear applications: Boron's ability to absorb neutrons makes it valuable in nuclear reactors as a control rod material and in radiation shielding.
- Abrasives: Boron compounds, such as boron carbide, possess exceptional hardness, making them ideal abrasives in industrial applications.
Liquid and Gaseous Boron: Specialized Applications
- Plasma processing: Boron's behavior in the plasma state is crucial in techniques like plasma-enhanced chemical vapor deposition (PECVD) for the synthesis of boron-containing thin films used in various electronic and optical devices.
- High-temperature applications: Although challenging to handle, research into liquid boron's properties is ongoing, exploring potential applications in extreme high-temperature environments.
Conclusion: The multifaceted nature of Boron
In conclusion, boron predominantly exists as a solid at standard temperature and pressure, exhibiting a complex crystalline structure. Its exceptionally high melting point, resulting from strong covalent bonding within its icosahedral units, necessitates extremely high temperatures to achieve the liquid phase. The gaseous state is less common, often observed through sublimation at very high temperatures or in plasma conditions. The study of boron's phase transitions continues to be an active area of research, with potential applications across numerous fields. Understanding boron's diverse states is vital for unlocking its full potential in advanced materials science, electronics, and various high-temperature applications. Further research promises to reveal more about this fascinating element's behavior under extreme conditions.
Latest Posts
Latest Posts
-
5 4 On A Number Line
Mar 22, 2025
-
What Is The Fraction For 0 9
Mar 22, 2025
-
How Far Is Earth From Pluto In Light Years
Mar 22, 2025
-
Is 3 4 Equal To 6 8
Mar 22, 2025
-
How Many Suns Are In The Universe
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Is Boron Solid Liquid Or Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
