Is Freezing A Chemical Or Physical Change
listenit
Mar 21, 2025 · 5 min read
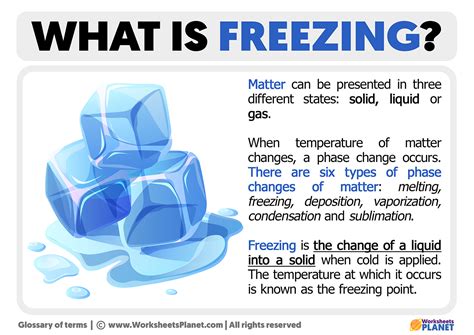
Table of Contents
Is Freezing a Chemical or Physical Change? A Deep Dive into States of Matter
The question of whether freezing is a chemical or physical change is a fundamental one in science, particularly when exploring the properties of matter and its transformations. While seemingly simple, the answer requires a nuanced understanding of the differences between chemical and physical changes and the underlying molecular behavior involved in the freezing process. This article will delve into the intricacies of this topic, exploring the key distinctions between chemical and physical changes, examining the process of freezing at a molecular level, and dispelling common misconceptions. We'll also touch upon related phase transitions and their classifications.
Understanding the Difference: Chemical vs. Physical Changes
Before we can definitively categorize freezing, it's crucial to understand the defining characteristics of chemical and physical changes.
Chemical Changes: Breaking and Making Bonds
A chemical change, also known as a chemical reaction, involves the alteration of the chemical composition of a substance. This means the arrangement of atoms within molecules is rearranged, forming new substances with different properties. Key indicators of a chemical change include:
- Formation of a new substance: The products possess distinctly different characteristics from the reactants.
- Irreversibility (usually): While some chemical reactions are reversible under specific conditions, many are not easily reversed.
- Energy changes: Chemical reactions often involve the release or absorption of significant amounts of energy in the form of heat, light, or sound.
- Changes in color, odor, or texture: These are often, but not always, observable signs of a chemical reaction.
Examples include burning wood (forming ash and gases), rusting iron (forming iron oxide), and baking a cake (complex chemical reactions involving flour, eggs, and sugar).
Physical Changes: Altering Form, Not Composition
A physical change, on the other hand, affects only the physical properties of a substance, such as its shape, size, or state of matter. The chemical composition remains unchanged. The atoms and molecules themselves remain the same; only their arrangement or energy levels are altered. Examples of physical changes include:
- Melting ice: Ice (solid water) turns into liquid water, but the water molecules are still H₂O.
- Boiling water: Liquid water turns into water vapor (gas), but the molecules are still H₂O.
- Crushing a can: The shape of the can changes, but the metal remains the same.
- Dissolving sugar in water: The sugar appears to disappear, but its molecules are still present in the solution, and can be recovered by evaporation.
Freezing: A Detailed Molecular Examination
Now, let's focus on the process of freezing. When a liquid freezes, it transitions from a liquid state to a solid state. This transition is driven by a decrease in temperature, causing the kinetic energy of the molecules to decrease significantly.
Molecular Behavior During Freezing
In a liquid, molecules are relatively free to move past one another. They possess high kinetic energy and are not arranged in a fixed pattern. As the temperature decreases, the kinetic energy of the molecules diminishes. This reduced energy allows intermolecular forces (such as hydrogen bonds in water) to become dominant. These forces pull the molecules closer together, and they begin to form a more ordered, structured arrangement characteristic of a solid.
For water, this ordered arrangement takes the form of a hexagonal crystalline structure, with each water molecule forming hydrogen bonds with several neighboring molecules. This structure is less dense than the liquid state, which explains why ice floats on water. The crucial point is that the water molecules themselves remain unchanged; they are still H₂O. Only their arrangement and freedom of movement have been altered.
Why Freezing is a Physical Change
Because the chemical composition of the substance undergoing freezing remains unchanged, the process is classified as a physical change. No new chemical bonds are formed or broken during freezing; only the arrangement of existing molecules is altered. The substance retains its chemical identity throughout the phase transition. If we were to melt the ice again, we would obtain the same water we started with, confirming the unchanged chemical composition.
Phase Transitions and Their Classification
Freezing is one example of a phase transition, a process where a substance changes from one state of matter to another. These transitions are generally physical changes, as they don't alter the chemical composition. Other common phase transitions include:
- Melting: The transition from solid to liquid.
- Boiling/Vaporization: The transition from liquid to gas.
- Condensation: The transition from gas to liquid.
- Sublimation: The transition from solid directly to gas (e.g., dry ice).
- Deposition: The transition from gas directly to solid (e.g., frost formation).
All these transitions are typically classified as physical changes because they do not involve the breaking or formation of chemical bonds. The substance's chemical identity remains preserved.
Addressing Common Misconceptions
Several common misconceptions surround the nature of freezing. Let's clarify some of them:
-
Freezing as a "Chemical Reaction": Some might mistakenly view freezing as a chemical reaction because it involves a change in state and energy transfer. However, the absence of bond breaking or formation clearly distinguishes it as a physical change.
-
Changes in Appearance as Evidence of Chemical Change: The altered appearance of a substance upon freezing (from liquid to solid) is a physical change, not a chemical one. The underlying chemical composition remains unaltered.
-
Ice vs. Water: Different Substances? Ice and liquid water are different phases of the same substance, water (H₂O). Their distinct physical properties (density, hardness, etc.) stem from the arrangement of their molecules, not from a change in their chemical composition.
Conclusion: Freezing – A Definitive Physical Change
In conclusion, freezing is unequivocally a physical change. The process involves a rearrangement of molecules due to a decrease in kinetic energy, leading to a more ordered, solid structure. No new substances are formed, and the chemical identity of the substance remains unchanged. While energy changes and alterations in physical properties occur, these are characteristic of physical, not chemical, processes. Understanding this distinction is fundamental to grasping the basic principles of chemistry and physics. By clarifying this, we solidify the understanding of phase transitions and the behavior of matter in its different states.
Latest Posts
Latest Posts
-
What Is 9 Oz In Cups
Mar 22, 2025
-
Greatest Common Factor Of 36 And 60
Mar 22, 2025
-
How Many Protons Does K Have
Mar 22, 2025
-
Rotation 180 Degrees Counter Clockwise About The Origin
Mar 22, 2025
-
Number Of Valence Electrons For Calcium
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Is Freezing A Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
