How Many Protons Does K Have
listenit
Mar 22, 2025 · 5 min read
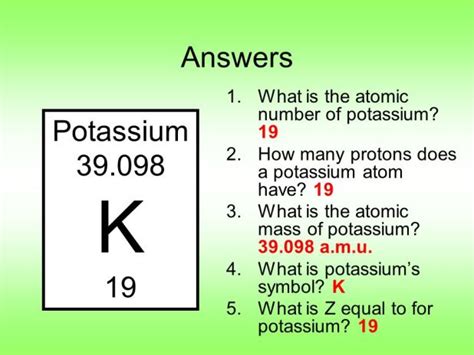
Table of Contents
How Many Protons Does K (Potassium) Have? Exploring Atomic Structure and Isotopes
Potassium, a vital element for life, holds a significant place in the periodic table. Understanding its atomic structure, specifically the number of protons it possesses, is fundamental to comprehending its chemical behavior and biological role. This in-depth exploration will delve into the answer to the question: How many protons does K have? We'll also explore related concepts like atomic number, isotopes, and the significance of potassium in various fields.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we answer the central question, let's establish a firm understanding of atomic structure. An atom, the basic building block of matter, consists of three primary subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element's identity and is crucial in determining its chemical properties.
- Neutrons: Neutrally charged particles also located in the nucleus. They contribute to the atom's mass but not its charge. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles that orbit the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
The Atomic Number: Defining the Element
The atomic number of an element is the number of protons in the nucleus of a single atom of that element. This number is unique to each element and is found on the periodic table. It's a fundamental property that distinguishes one element from another. Elements are arranged on the periodic table in ascending order of their atomic numbers.
This is where we find the answer to our primary question. Potassium (K) has an atomic number of 19. This unequivocally means that every potassium atom contains 19 protons. This is a fundamental truth about potassium and its atomic identity.
Isotopes: Variations in Neutron Count
While the number of protons defines an element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. Isotopes of an element have the same atomic number (same number of protons) but different mass numbers (total number of protons and neutrons).
Potassium has several naturally occurring isotopes, the most common being:
- Potassium-39 (³⁹K): This is the most abundant isotope, comprising about 93.3% of naturally occurring potassium. It has 19 protons and 20 neutrons.
- Potassium-40 (⁴⁰K): A radioactive isotope, comprising about 0.012% of naturally occurring potassium. It has 19 protons and 21 neutrons. This isotope is significant in geological dating and is a source of background radiation.
- Potassium-41 (⁴¹K): This stable isotope makes up approximately 6.7% of naturally occurring potassium. It contains 19 protons and 22 neutrons.
It's important to remember that despite having different numbers of neutrons, all isotopes of potassium have the same number of protons – 19. This consistent proton count is what makes them all potassium.
The Significance of Potassium (K)
Potassium's importance extends far beyond its atomic structure. It plays crucial roles in various fields:
Biological Roles:
- Maintaining Fluid Balance: Potassium is essential for regulating the balance of fluids inside and outside cells. This is critical for nerve and muscle function.
- Nerve and Muscle Function: Potassium ions (K⁺) are involved in the transmission of nerve impulses and muscle contractions. Imbalances can lead to serious health problems.
- Enzyme Activity: Potassium acts as a cofactor for numerous enzymes, assisting in various metabolic processes within the body.
- Blood Pressure Regulation: Potassium plays a role in regulating blood pressure. A proper potassium intake is linked to healthy blood pressure levels.
Potassium deficiency (hypokalemia) can lead to muscle weakness, fatigue, irregular heartbeat, and other health issues. Conversely, excessively high potassium levels (hyperkalemia) can also be dangerous.
Industrial Applications:
- Fertilizers: Potassium is a vital component of fertilizers, supplying essential nutrients for plant growth. Potassium-rich fertilizers are crucial for optimal crop yields.
- Glass Manufacturing: Potassium compounds are used in the manufacturing of certain types of glass, enhancing its properties.
- Soap Production: Potassium hydroxide (KOH) is used in the production of soft soaps.
Geological Significance:
- Geological Dating: The radioactive isotope potassium-40 is used in radiometric dating techniques to determine the age of rocks and minerals. This provides insights into Earth's history and geological processes.
Further Exploration of Atomic Structure and Isotopes
Understanding the atomic structure, specifically the number of protons, is fundamental to comprehending the behavior of elements. The concept of isotopes highlights that while the number of protons defines an element, variations in neutron count can lead to differences in mass and radioactive properties.
The case of potassium exemplifies the interplay between atomic structure and its implications in diverse fields, from biological processes to industrial applications and geological dating. Further investigation into the intricacies of atomic structure and isotopic variations will continue to deepen our understanding of the material world and its fundamental constituents.
Conclusion: The Defining Number of Protons in Potassium
In summary, the answer to the question "How many protons does K have?" is 19. This atomic number definitively identifies the element as potassium and governs its chemical and physical properties. This seemingly simple number holds immense significance in understanding the element's biological role, its industrial applications, and its contribution to various scientific disciplines. The exploration of potassium's isotopes further enriches our knowledge of atomic structure and its implications in the natural world. The consistent presence of 19 protons within every potassium atom is the defining characteristic of this essential element.
Latest Posts
Latest Posts
-
What Is 12 5 In Fraction Form
Mar 22, 2025
-
Is Diameter The Same As Radius
Mar 22, 2025
-
What Is 20 As A Percentage Of 50
Mar 22, 2025
-
Convert 89 Degrees Fahrenheit To Celsius
Mar 22, 2025
-
How Many Electrons Can The Second Shell Hold
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Does K Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
