How Many Electrons Can The Second Shell Hold
listenit
Mar 22, 2025 · 6 min read
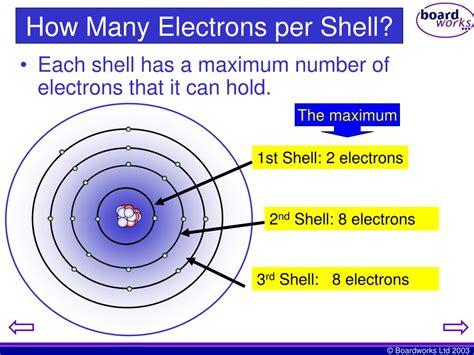
Table of Contents
How Many Electrons Can the Second Shell Hold? A Deep Dive into Atomic Structure
Understanding the arrangement of electrons within an atom is fundamental to comprehending chemistry and physics. This article delves into the specifics of electron shell capacity, focusing particularly on the second electron shell. We'll explore the underlying principles governing electron distribution, the implications for chemical bonding, and the fascinating exceptions that highlight the complexities of quantum mechanics.
The Basics: Electron Shells and Subshells
Atoms consist of a nucleus containing protons and neutrons, surrounded by orbiting electrons. These electrons don't orbit randomly; they occupy specific energy levels, often visualized as concentric shells around the nucleus. The first shell, closest to the nucleus, has the lowest energy level. Subsequent shells have progressively higher energy levels.
The number of electrons a shell can hold is determined by a simple formula: 2n², where 'n' is the principal quantum number representing the shell's energy level. For the first shell (n=1), this means a maximum of 2(1)² = 2 electrons. For the second shell (n=2), the formula yields 2(2)² = 8 electrons. This is the key answer to our central question: the second electron shell can hold a maximum of eight electrons.
However, the story doesn't end there. Each shell is further divided into subshells, characterized by different shapes and energy levels. These subshells are crucial in understanding the detailed arrangement of electrons and their behavior.
Subshells and Orbitals: Unveiling the Electron's Home
Within each shell, subshells are identified by letters: s, p, d, and f. The s subshell is spherical, the p subshell has a dumbbell shape, and the d and f subshells are more complex. Each subshell can hold a specific number of electrons:
- s subshell: Holds a maximum of 2 electrons.
- p subshell: Holds a maximum of 6 electrons.
- d subshell: Holds a maximum of 10 electrons.
- f subshell: Holds a maximum of 14 electrons.
The second shell contains only the s and p subshells. Therefore, its total capacity of eight electrons is distributed as follows: two electrons in the 2s subshell and six electrons in the 2p subshell (2 + 6 = 8).
The Significance of the Octet Rule
The capacity of the second shell to hold eight electrons is deeply connected to the octet rule. This fundamental principle in chemistry states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight electrons in their outermost shell (valence shell). This stable configuration resembles the electron arrangement of noble gases, which are exceptionally unreactive.
Achieving an octet often leads to the formation of chemical bonds. Atoms will readily interact with other atoms to either gain, lose, or share electrons and attain this stable octet structure. This is the driving force behind many chemical reactions.
How the Octet Rule Works with the Second Shell
Consider the element oxygen (O), with an atomic number of 8. Its electron configuration is 1s²2s²2p⁴. This means it has two electrons in the first shell and six electrons in the second shell. To achieve a stable octet, oxygen needs to gain two more electrons. This often happens through the formation of covalent bonds, where it shares electrons with other atoms.
Similarly, sodium (Na), with an atomic number of 11, has an electron configuration of 1s²2s²2p⁶3s¹. To achieve a stable octet, it readily loses the single electron in its third shell, leaving it with a full second shell (1s²2s²2p⁶). This loss of an electron leads to the formation of a positive ion (Na⁺).
Beyond the Octet Rule: Exceptions and Complexities
While the octet rule is a useful guideline, it's not without exceptions. Some atoms, particularly those in the third row of the periodic table and beyond, can accommodate more than eight electrons in their valence shell due to the availability of d orbitals. This phenomenon is often observed in transition metals and post-transition metals.
Expanded Octet: When More Than Eight Electrons is Possible
Elements like phosphorus (P) and sulfur (S) can form compounds where their valence shells have more than eight electrons. This happens because the d orbitals in the third shell and beyond can participate in bonding. Such molecules violate the octet rule but are nevertheless stable. This underscores the fact that the octet rule is a helpful simplification rather than an absolute law.
The Role of Quantum Mechanics
The precise arrangement of electrons in atoms is governed by the principles of quantum mechanics. The concept of electron shells and subshells arises from the solution of the Schrödinger equation, which describes the behavior of electrons in atoms. Quantum numbers, including the principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms), dictate the allowed energy levels and the spatial distribution of electrons within an atom.
The Pauli Exclusion Principle is a cornerstone of quantum mechanics, stating that no two electrons in an atom can have the same set of four quantum numbers. This principle limits the number of electrons that can occupy a particular orbital to a maximum of two, with opposite spins. This principle ensures that the electron configuration within the second shell follows the 2s²2p⁶ arrangement.
Applications and Further Exploration
Understanding the electron configuration of atoms, specifically the capacity of the second shell, is critical in various scientific fields:
- Chemistry: Predicting chemical bonding, reactivity, and the properties of compounds.
- Materials Science: Designing materials with specific electrical, magnetic, and optical properties.
- Physics: Understanding atomic spectra and the interaction of atoms with light and other forms of radiation.
- Biology: Understanding the structure and function of biomolecules and their interactions.
This knowledge forms the basis for more advanced concepts in chemistry and physics, including molecular orbital theory, valence bond theory, and the periodic trends observed in the elements.
Conclusion: The Second Shell and its Crucial Role
The second electron shell's capacity to hold eight electrons is a cornerstone principle in chemistry. Its influence extends far beyond simple calculations, shaping the reactivity of elements, the formation of chemical bonds, and the properties of countless molecules. While exceptions and complexities exist, the fundamental concept remains a powerful tool for understanding the behavior of matter at the atomic level. Further exploration into quantum mechanics reveals the intricate rules governing electron distribution, highlighting the elegant yet complex nature of atomic structure. The second shell, seemingly simple in its eight-electron capacity, provides a vital window into the fascinating world of atomic physics and chemistry.
Latest Posts
Latest Posts
-
Is Mars A Terrestrial Or Gaseous Planet
May 09, 2025
-
Where Does Transcription Occur In A Prokaryotic Cell
May 09, 2025
-
The Sum Of A Number And 3
May 09, 2025
-
What Is The Name Of The Molecular Compound So3
May 09, 2025
-
What Is 70 F To Celsius
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can The Second Shell Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.