Number Of Valence Electrons For Calcium
listenit
Mar 22, 2025 · 6 min read
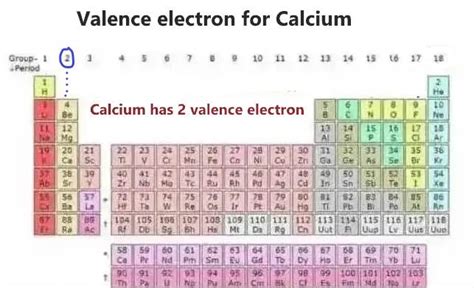
Table of Contents
Unveiling the Secrets of Calcium's Valence Electrons: A Deep Dive
Calcium, a vital element for life, plays a crucial role in various biological processes. Understanding its electronic structure, particularly its valence electrons, is key to comprehending its chemical behavior and reactivity. This comprehensive article delves into the intricacies of calcium's valence electrons, exploring its electronic configuration, bonding properties, and significance in biological systems. We'll unravel the mystery behind why calcium behaves the way it does, using a blend of theoretical concepts and practical applications.
Understanding Valence Electrons: The Key to Chemical Reactivity
Before focusing specifically on calcium, let's establish a foundational understanding of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. They are the primary players in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons dictates the element's position in the periodic table and its preferred oxidation states. Atoms strive for stability, often achieving this by gaining, losing, or sharing valence electrons to attain a full outermost shell (usually eight electrons, following the octet rule, except for hydrogen and helium).
Calcium's Electronic Configuration: A Closer Look
Calcium (Ca) sits proudly in Group 2 (alkaline earth metals) of the periodic table, with an atomic number of 20. This means a neutral calcium atom has 20 protons and 20 electrons. To understand its valence electrons, we need to delve into its electronic configuration: 1s²2s²2p⁶3s²3p⁶4s².
This configuration reveals a structured arrangement of electrons within different energy levels (shells) and sublevels (subshells). The numbers (1, 2, 3, 4) represent the principal quantum numbers, indicating the energy level. The letters (s, p) denote the subshells, each capable of holding a specific number of electrons.
Deciphering the Electronic Configuration
- 1s²: Two electrons in the first energy level's s subshell.
- 2s²2p⁶: Two electrons in the second energy level's s subshell and six in the p subshell, totaling eight electrons filling the second shell.
- 3s²3p⁶: Similar to the second shell, this level holds eight electrons, filling the third shell completely.
- 4s²: Crucially, this is where we find calcium's valence electrons. Two electrons reside in the fourth energy level's s subshell.
Calcium's Valence Electrons: The Number and Their Significance
From the electronic configuration, it's crystal clear: calcium has two valence electrons. These are the electrons most readily involved in chemical reactions. Their presence in the outermost shell makes them loosely held compared to inner electrons, susceptible to being lost or shared during chemical interactions.
The significance of these two valence electrons is profound:
- Reactivity: Calcium's tendency to lose its two valence electrons to achieve a stable, noble gas configuration (similar to Argon) makes it highly reactive, particularly with nonmetals and electronegative elements.
- Ionic Bonding: Losing these two electrons leads to the formation of a Ca²⁺ ion, a stable cation with a +2 charge. This ionic form is crucial in numerous chemical reactions and biological processes.
- Metallic Bonding: In its metallic state, calcium's valence electrons participate in metallic bonding, a type of bonding where valence electrons are delocalized and shared amongst a "sea" of electrons, contributing to calcium's characteristic metallic properties such as conductivity and malleability.
- Oxidation State: Calcium almost exclusively exhibits a +2 oxidation state due to its tendency to readily lose its two valence electrons. This consistency makes predicting its behavior in chemical reactions relatively straightforward.
Calcium's Chemical Behavior: A Consequence of its Valence Electrons
The two valence electrons in calcium dictate its chemical behavior in numerous ways:
- Reaction with Water: Calcium reacts readily with water to produce calcium hydroxide (Ca(OH)₂) and hydrogen gas (H₂). This reaction demonstrates its strong tendency to lose its valence electrons.
- Reaction with Oxygen: Calcium burns readily in air, forming calcium oxide (CaO), another testament to its high reactivity. This reaction involves the loss of its two valence electrons to oxygen atoms.
- Formation of Ionic Compounds: Calcium readily forms ionic compounds with nonmetals, such as calcium chloride (CaCl₂), calcium fluoride (CaF₂), and calcium sulfate (CaSO₄). In these compounds, calcium exists as the Ca²⁺ ion, a direct consequence of its loss of two valence electrons.
- Role as a Reducing Agent: Because of its willingness to lose electrons, calcium functions effectively as a reducing agent in many chemical reactions, facilitating the reduction of other elements by donating electrons.
Calcium in Biological Systems: The Role of its Valence Electrons
Calcium's two valence electrons are instrumental in its crucial biological roles:
- Bone Formation: Calcium ions (Ca²⁺) are essential components of bones and teeth, forming strong and rigid structures. The ionic bonds between calcium and other ions, particularly phosphate ions, contribute to the structural integrity of bone tissue.
- Muscle Contraction: Calcium ions play a critical regulatory role in muscle contraction. The precise movement of calcium ions within muscle cells triggers the interaction of muscle proteins, leading to muscle contraction and relaxation.
- Nerve Impulse Transmission: Calcium ions are involved in the transmission of nerve impulses, facilitating communication between nerve cells. This process relies on the controlled movement and interaction of calcium ions across nerve cell membranes.
- Blood Clotting: Calcium ions are essential for blood clotting. They participate in a cascade of reactions involving various clotting factors, ultimately leading to the formation of a blood clot to prevent excessive bleeding.
- Enzyme Activation: Many enzymes require calcium ions for their proper functioning. Calcium ions act as cofactors, aiding the enzyme's catalytic activity.
Comparing Calcium with Other Group 2 Elements: Similarities and Differences
Calcium's chemical behavior mirrors that of other Group 2 elements (alkaline earth metals) such as beryllium (Be), magnesium (Mg), strontium (Sr), barium (Ba), and radium (Ra). They all share the characteristic of having two valence electrons and readily forming +2 ions. However, subtle differences arise due to variations in atomic size and electronegativity. Calcium's reactivity, for instance, is less pronounced than that of the more reactive alkaline earth metals like barium, reflecting the influence of its atomic size and electronegativity.
Conclusion: The Importance of Understanding Calcium's Valence Electrons
Understanding the number and significance of calcium's valence electrons (two) is paramount for comprehending its chemical reactivity, bonding properties, and crucial biological roles. Its tendency to lose these two electrons drives its chemical behavior, leading to the formation of ionic compounds, its participation in various biological processes, and its role as a reducing agent. The interplay between its electronic structure and chemical behavior underscores the fundamental link between atomic structure and macroscopic properties, a crucial concept in chemistry and biochemistry. This deep dive into calcium's valence electrons not only illuminates its inherent chemical properties but also reveals its indispensable role in supporting life as we know it. The study of valence electrons, therefore, extends beyond abstract chemical concepts, providing a vital key to understanding the intricate functioning of biological systems and the world around us.
Latest Posts
Latest Posts
-
What Is 12 5 In Fraction Form
Mar 22, 2025
-
Is Diameter The Same As Radius
Mar 22, 2025
-
What Is 20 As A Percentage Of 50
Mar 22, 2025
-
Convert 89 Degrees Fahrenheit To Celsius
Mar 22, 2025
-
How Many Electrons Can The Second Shell Hold
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons For Calcium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
