What Do Coefficients Represent In A Chemical Equation
listenit
Mar 31, 2025 · 6 min read
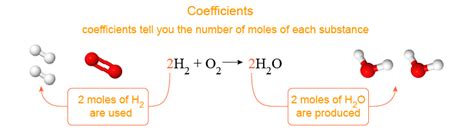
Table of Contents
What Do Coefficients Represent in a Chemical Equation?
Coefficients in a chemical equation are numerical values placed before chemical formulas. They represent the relative number of moles of reactants and products involved in a balanced chemical reaction. Understanding what coefficients represent is fundamental to comprehending stoichiometry, a crucial aspect of chemistry dealing with quantitative relationships between reactants and products in chemical reactions. This article delves into the significance of coefficients, explaining their role in balancing equations, calculating molar ratios, and predicting reaction yields. We will also explore how coefficients relate to other chemical concepts, such as limiting reactants and percent yield.
Understanding Chemical Equations
Before we dive into coefficients, let's establish a clear understanding of chemical equations. A chemical equation is a symbolic representation of a chemical reaction. It uses chemical formulas to depict the reactants (starting substances) and products (substances formed) involved. For instance:
2H₂ + O₂ → 2H₂O
This equation represents the reaction between hydrogen gas (H₂) and oxygen gas (O₂) to produce water (H₂O). The arrow (→) indicates the direction of the reaction, from reactants to products.
The Role of Coefficients in Balancing Equations
Chemical equations must be balanced to adhere to the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. Balancing ensures that the number of atoms of each element is the same on both sides of the equation. Coefficients are used to achieve this balance.
In the equation above:
- 2H₂: indicates two molecules of hydrogen gas, contributing a total of four hydrogen atoms (2 x 2 = 4).
- O₂: indicates one molecule of oxygen gas, contributing two oxygen atoms.
- 2H₂O: indicates two molecules of water, contributing four hydrogen atoms (2 x 2 = 4) and two oxygen atoms.
The coefficients (2, 1, and 2) ensure that the number of hydrogen and oxygen atoms is equal on both the reactant and product sides. Balancing equations is a trial-and-error process, and while there are systematic approaches, practice is key to mastering the skill.
Importance of Balanced Equations
Balanced chemical equations are crucial for several reasons:
- Accurate Stoichiometric Calculations: Coefficients provide the molar ratios between reactants and products, essential for calculating the amounts of reactants needed or products formed in a reaction.
- Predicting Reaction Outcomes: A balanced equation allows us to predict the quantities of products that can be formed from a given amount of reactants, helping in experimental design and industrial processes.
- Understanding Reaction Mechanisms: Although not directly evident from the balanced equation itself, the coefficients can offer insights into the reaction's mechanism, suggesting possible intermediate steps involved.
Coefficients and Molar Ratios
The coefficients in a balanced chemical equation directly represent the molar ratios of the substances involved. Molar ratio refers to the ratio of the number of moles of one substance to the number of moles of another substance in a chemical reaction.
In the reaction:
2H₂ + O₂ → 2H₂O
The molar ratio of H₂ to O₂ is 2:1. This means that for every 2 moles of hydrogen gas reacting, 1 mole of oxygen gas is required. Similarly, the molar ratio of H₂ to H₂O is 2:2 (or 1:1), and the molar ratio of O₂ to H₂O is 1:2.
These molar ratios are fundamental for solving stoichiometry problems. They allow us to calculate the amount of one substance needed or produced based on the known amount of another substance.
Coefficients, Limiting Reactants, and Percent Yield
Coefficients play a vital role in determining the limiting reactant in a chemical reaction. The limiting reactant is the reactant that is completely consumed first, thus limiting the amount of product that can be formed. Identifying the limiting reactant requires comparing the molar ratios of reactants to their actual amounts provided.
Furthermore, coefficients contribute to calculating the percent yield of a reaction. Percent yield represents the ratio of the actual yield (the amount of product obtained experimentally) to the theoretical yield (the amount of product expected based on stoichiometric calculations using the coefficients) multiplied by 100%. A low percent yield might indicate incomplete reaction or side reactions occurring.
Advanced Concepts and Applications
The understanding of coefficients extends to more complex chemical situations, such as:
- Reactions involving multiple reactants and products: Coefficients help balance equations with numerous substances, ensuring the conservation of mass principle.
- Reactions in aqueous solutions: Coefficients are equally vital when dealing with reactions occurring in solutions, particularly in acid-base titrations and precipitation reactions.
- Gas stoichiometry: Coefficients are critical when calculating gas volumes involved in a reaction using the ideal gas law, considering the molar relationships determined by the coefficients.
- Thermochemistry: Coefficients are used in thermochemical calculations, as they determine the amount of heat transferred (enthalpy change) in a reaction.
Examples Illustrating Coefficient Significance
Let's consider a few more examples to solidify our understanding:
Example 1: Combustion of Methane
CH₄ + 2O₂ → CO₂ + 2H₂O
This equation represents the combustion of methane (CH₄) in oxygen (O₂), producing carbon dioxide (CO₂) and water (H₂O). The coefficient 2 before O₂ signifies that two moles of oxygen are required to react with one mole of methane. The coefficients also reveal that one mole of methane produces one mole of carbon dioxide and two moles of water.
Example 2: Formation of Ammonia
N₂ + 3H₂ → 2NH₃
This equation represents the synthesis of ammonia (NH₃) from nitrogen (N₂) and hydrogen (H₂). The coefficients 1, 3, and 2 indicate the molar ratios needed for the reaction to proceed and the amounts produced. Three moles of hydrogen are required for every mole of nitrogen, leading to the formation of two moles of ammonia.
Example 3: A More Complex Reaction
2FeCl₃ + 3H₂S → Fe₂S₃ + 6HCl
This equation involves multiple reactants and products. Coefficients ensure that the number of iron (Fe), chlorine (Cl), hydrogen (H), and sulfur (S) atoms remains consistent on both sides. The balanced equation allows calculations for yields based on the molar amounts of reactants used.
Conclusion
Coefficients in chemical equations are more than just numbers; they are fundamental to understanding the quantitative aspects of chemical reactions. They represent the relative number of moles of reactants and products, enabling accurate stoichiometric calculations, prediction of reaction yields, and identification of limiting reactants. Mastering the concept of coefficients is essential for anyone seeking a deeper understanding of chemistry, its principles, and its practical applications. From basic stoichiometry problems to advanced topics like thermochemistry and reaction kinetics, the significance of coefficients remains paramount in the realm of chemical sciences. Continued practice and problem-solving are key to solidifying this crucial concept and applying it effectively.
Latest Posts
Latest Posts
-
How Do You Find The Slope Of A Line Perpendicular
Apr 02, 2025
-
Divide 2x2 17x 35 By X 5
Apr 02, 2025
-
Number Of Valence Electrons For Carbon
Apr 02, 2025
-
How To Find Density Of A Solution
Apr 02, 2025
-
What Is The Gcf Of 45 And 75
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Do Coefficients Represent In A Chemical Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
