What Color Of Light Has The Highest Energy
listenit
Mar 31, 2025 · 5 min read
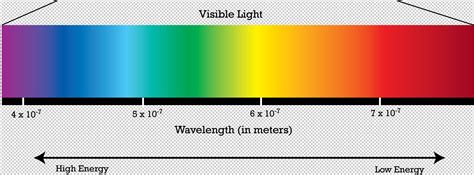
Table of Contents
What Color of Light Has the Highest Energy? Understanding the Electromagnetic Spectrum
The question of which color of light possesses the highest energy is fundamental to understanding the nature of light and its interaction with matter. It's a seemingly simple question with a surprisingly rich answer that delves into the fascinating world of the electromagnetic spectrum. This article will explore this topic in detail, explaining the relationship between color, wavelength, frequency, and energy, and ultimately answering the question definitively. We'll also touch upon the practical applications and implications of this understanding.
The Electromagnetic Spectrum: A Rainbow of Energy
Light, as we perceive it, is only a tiny sliver of a much broader spectrum of electromagnetic radiation. This spectrum encompasses a vast range of wavelengths and frequencies, each carrying different amounts of energy. From the longest wavelengths to the shortest, we find radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays.
Visible light, the portion we can see, is further broken down into the familiar colors of the rainbow: red, orange, yellow, green, blue, indigo, and violet. These colors are not arbitrary; they represent different wavelengths of light, and consequently, different energies.
Wavelength and Frequency: The Key Players
The energy of light is directly related to its wavelength and frequency. Wavelength refers to the distance between successive crests of a light wave, while frequency refers to the number of wave crests passing a given point per second. These two are inversely proportional: shorter wavelengths correspond to higher frequencies, and vice-versa.
The relationship is described by the following equation:
c = λν
Where:
cis the speed of light (approximately 3 x 10<sup>8</sup> meters per second)λ(lambda) is the wavelengthν(nu) is the frequency
The Energy-Frequency Relationship: Unveiling the Secret
The crucial link between these properties and energy is given by Planck's equation:
E = hν
Where:
Eis the energy of the lighthis Planck's constant (approximately 6.626 x 10<sup>-34</sup> joule-seconds)νis the frequency
This equation reveals that the energy of light is directly proportional to its frequency. Higher frequency light carries more energy. Since frequency and wavelength are inversely related, this also means that shorter wavelengths correspond to higher energy.
Which Color Has the Highest Energy? The Verdict
Combining our understanding of the visible spectrum and the energy-frequency relationship, we can definitively answer the question: violet light has the highest energy within the visible spectrum.
Violet light has the shortest wavelength and therefore the highest frequency among the colors we can see. This higher frequency translates directly to higher energy according to Planck's equation. Red light, on the other hand, has the longest wavelength and lowest frequency, resulting in the lowest energy within the visible range.
Beyond the Visible: Even Higher Energies
It's crucial to remember that visible light represents only a small fraction of the electromagnetic spectrum. Beyond violet light, we encounter ultraviolet (UV) radiation, X-rays, and gamma rays. These forms of radiation have even shorter wavelengths and higher frequencies than violet light, meaning they carry significantly more energy. Gamma rays, in particular, possess the highest energy of all electromagnetic radiation.
Practical Applications and Implications
The energy content of light has profound implications in various fields:
1. Photoelectric Effect: Harnessing Light's Energy
The photoelectric effect demonstrates the relationship between light energy and electron emission. When light of sufficient energy (above a certain threshold frequency) strikes a metal surface, it can eject electrons. This phenomenon is crucial in technologies such as photoelectric cells used in solar panels and light meters. Higher energy light, like UV, is more effective at this process.
2. Spectroscopy: Unraveling Atomic Structure
Spectroscopy analyzes the interaction of light with matter, providing insights into the composition and structure of materials. Different elements absorb and emit light at specific wavelengths, creating unique spectral "fingerprints." Analyzing these fingerprints helps identify the elements present in a sample. The energy of the light absorbed or emitted reflects the energy level transitions within the atoms.
3. Medical Applications: Diagnosis and Treatment
High-energy radiation, such as X-rays and gamma rays, is extensively used in medical imaging and therapy. X-rays are used in radiography to create images of bones and internal organs, while gamma rays are used in radiation therapy to target and destroy cancerous cells. The high energy of these rays allows them to penetrate tissues and interact with matter in specific ways.
4. Materials Science: Modifying Properties
The energy of light can be used to modify the properties of materials. For example, ultraviolet light can be used to initiate polymerization reactions, leading to the creation of new materials. Laser technology utilizes highly focused, monochromatic light, often in the UV or visible range, for precision material processing, including cutting, engraving, and surface modification.
5. Astronomy: Peering into the Cosmos
Astronomers use various wavelengths of electromagnetic radiation to study celestial objects. Different wavelengths reveal different aspects of these objects. For example, infrared radiation can penetrate dust clouds, allowing astronomers to observe stars and galaxies hidden from view in the visible spectrum. Analyzing the energy distribution of light from distant sources helps determine their composition, temperature, and distance.
Understanding the Nuances: Beyond Simple Color
While violet light has the highest energy within the visible spectrum, it's important to note that the energy of light is not solely determined by its perceived color. Factors like intensity (the brightness of the light) also play a crucial role. A bright red light can deliver more total energy than a dim violet light, even though each individual photon of violet light has more energy.
Conclusion: A Deeper Dive into Light's Power
The relationship between light's color, wavelength, frequency, and energy is a cornerstone of physics and has far-reaching consequences in diverse fields. While violet light holds the title of highest energy within the visible spectrum, the electromagnetic spectrum extends far beyond visible light, encompassing radiation with far greater energy levels. Understanding this fundamental relationship allows us to harness the power of light for technological advancements and scientific discoveries, continuing to illuminate our understanding of the universe. The exploration continues, with new discoveries and applications constantly emerging, fueled by our ongoing quest to understand the intricate nature of light and its energy.
Latest Posts
Latest Posts
-
What Percent Of 50 Is 9
Apr 01, 2025
-
40 Is 60 Percent Of What Number
Apr 01, 2025
-
What Is The Conjugate Acid Of Hco3
Apr 01, 2025
-
Indicate A Condensed Structural Formula For The Following Compound
Apr 01, 2025
-
What Subatomic Particle Determines The Identity Of An Atom
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Color Of Light Has The Highest Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
