Indicate A Condensed Structural Formula For The Following Compound
listenit
Apr 01, 2025 · 5 min read
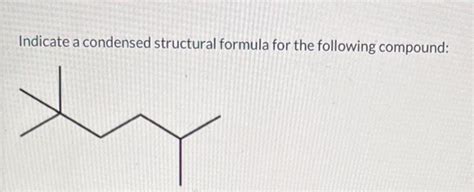
Table of Contents
Indicating Condensed Structural Formulas: A Comprehensive Guide
Condensed structural formulas are a powerful tool for representing organic molecules in a compact yet informative way. Unlike fully drawn structural formulas, which can become cumbersome for larger molecules, condensed formulas prioritize clarity and efficiency. This guide delves into the intricacies of condensed structural formulas, providing a step-by-step approach to constructing them and highlighting their importance in organic chemistry. We will cover various examples, addressing common challenges and misconceptions.
Understanding the Basics of Condensed Structural Formulas
A condensed structural formula shows the arrangement of atoms in a molecule but without explicitly drawing all the bonds. It implies the connectivity of atoms through the sequence in which they are written. Carbon atoms are usually not explicitly written, but are implied. Hydrogen atoms bonded to carbon are written after the carbon atom they are attached to. Other atoms or groups are written directly attached to the carbon they are bound to.
Key Features:
- Carbon Atoms Implicit: Carbon atoms form the backbone of most organic molecules. In a condensed formula, the carbon atoms are often implied, and their presence is inferred from the arrangement of other atoms.
- Hydrogen Atoms Attached to Carbon: Hydrogen atoms bonded to carbon atoms are listed after the carbon atom to which they are attached. For example,
CH₃represents a methyl group (one carbon atom bonded to three hydrogen atoms). - Other Atoms Explicitly Shown: All other atoms (e.g., oxygen, nitrogen, halogens) are explicitly shown in the formula and their positions relative to the carbon chain indicated.
- Parentheses Indicate Branching: Parentheses are used to show branching points in the molecule. Atoms or groups within parentheses are attached to the atom immediately preceding the opening parenthesis.
Step-by-Step Guide to Constructing Condensed Structural Formulas
Let's break down the process of creating condensed structural formulas using examples of increasing complexity.
Example 1: Simple Alkanes
Let's consider propane (C₃H₈). The fully drawn structural formula is:
H H H
| | |
H-C-C-C-H
| | |
H H H
The condensed structural formula simplifies this to: CH₃CH₂CH₃
Here, we imply that each carbon atom has the necessary hydrogen atoms to satisfy its valency (four bonds).
Example 2: Introducing Branching
Consider isobutane (C₄H₁₀). The fully drawn structural formula is:
CH₃
|
H₃C-C-CH₃
|
H
The condensed structural formula is: (CH₃)₂CHCH₃ The parentheses show that two methyl groups (CH₃) are attached to the central carbon atom.
Example 3: Incorporating Functional Groups
Let's examine ethanol (C₂H₅OH). Its fully drawn structure is:
H H
| |
H-C-C-O-H
| |
H H
The condensed structural formula is: CH₃CH₂OH. The hydroxyl group (-OH) is explicitly shown.
Example 4: More Complex Molecules
Consider 2-methylpentane. The expanded structure is:
CH₃
|
CH₃-CH-CH₂-CH₂-CH₃
The condensed structural formula is: CH₃CH(CH₃)CH₂CH₂CH₃. The parenthesis shows a methyl group attached to the second carbon atom.
Example 5: Molecules with Multiple Functional Groups
Let's consider a molecule with multiple functional groups, such as 2-chloro-3-hydroxybutanoic acid:
The expanded structure (though still simplified) is quite complex; a condensed formula provides much needed clarity: CH₃CH(OH)CH(Cl)COOH. This clearly shows the positions of the chlorine and hydroxyl groups on the butanoic acid backbone.
Common Mistakes to Avoid
- Forgetting Implicit Carbons: Remember that carbon atoms are often implied, particularly in longer chains. Don't omit them accidentally.
- Incorrect Placement of Parentheses: The parentheses must correctly indicate the branching points. Incorrect placement changes the meaning of the molecule.
- Ignoring Valency: Ensure that each atom (except hydrogen) has the correct number of bonds.
Applications of Condensed Structural Formulas
Condensed structural formulas are invaluable in various aspects of organic chemistry:
- Nomenclature: They are frequently used in naming organic compounds, aiding in the systematic assignment of IUPAC names.
- Reaction Mechanisms: They help visualize the changes in molecular structure during chemical reactions, making it easier to follow the flow of electrons and the formation/breaking of bonds.
- Spectroscopy: Condensed formulas assist in interpreting spectroscopic data, like NMR and IR spectroscopy, by providing a clear picture of the molecule's structure.
- Drug Design: In pharmaceutical chemistry, condensed formulas offer a compact yet informative way to represent complex drug molecules.
Advanced Techniques and Considerations
While the basic principles outlined above cover a wide range of molecules, certain situations require additional considerations:
- Cyclic Compounds: Cyclic compounds need to be carefully represented, often using shorthand notation to indicate the ring structure. For example, cyclohexane is often represented as
(CH₂)₆. - Aromatic Compounds: Benzene and its derivatives often employ a simplified representation of the aromatic ring, typically a hexagon with a circle inside.
- Stereochemistry: Condensed formulas generally don't explicitly represent stereochemistry (e.g., chirality). Additional notations are often needed to show spatial arrangements of atoms.
Conclusion
Condensed structural formulas provide an efficient and concise way to represent the structure of organic molecules. Mastering their construction is a crucial skill for any student or professional working with organic chemistry. By understanding the underlying principles and practicing with a variety of examples, you can confidently use condensed formulas to depict complex molecules, simplifying their visualization and aiding in various chemical tasks. Remember to always double-check your work, ensuring that valency rules are satisfied and that the connectivity is correctly reflected. The ability to confidently translate between full structural formulas and condensed formulas is a hallmark of proficiency in organic chemistry. The practice and understanding of these techniques will prove invaluable in your further studies and applications.
Latest Posts
Latest Posts
-
How To Calculate Mass Of Solute
Apr 02, 2025
-
Ice Will Melt Spontaneously At A Certain Temperature If
Apr 02, 2025
-
How To Find Concentration From Dilution
Apr 02, 2025
-
What Is The Equivalent Fraction Of 3 8
Apr 02, 2025
-
Limit Of X As X Approaches 0
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Indicate A Condensed Structural Formula For The Following Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
