What Subatomic Particle Determines The Identity Of An Atom
listenit
Apr 01, 2025 · 5 min read
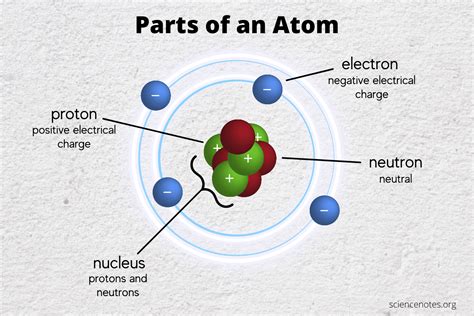
Table of Contents
What Subatomic Particle Determines the Identity of an Atom?
The identity of an atom, its unique place on the periodic table, is fundamentally determined by a single subatomic particle: the proton. While electrons and neutrons play crucial roles in an atom's properties and behavior, it's the number of protons that defines which element an atom belongs to. This number, known as the atomic number, is the cornerstone of atomic structure and chemistry.
Understanding the Subatomic Particles
Before diving into the central role of protons, let's briefly revisit the three primary subatomic particles:
1. Protons
- Charge: Positive (+)
- Mass: Approximately 1 atomic mass unit (amu)
- Location: Nucleus
- Key Role: Determines the atomic number and thus the element's identity. The number of protons is unique to each element and never changes within a stable atom.
2. Neutrons
- Charge: Neutral (0)
- Mass: Approximately 1 amu
- Location: Nucleus
- Key Role: Contributes to the atom's mass and stability. Isotopes, atoms of the same element with differing numbers of neutrons, demonstrate the impact of neutrons on atomic properties like radioactivity.
3. Electrons
- Charge: Negative (-)
- Mass: Negligible compared to protons and neutrons (approximately 1/1836 amu)
- Location: Electron cloud surrounding the nucleus
- Key Role: Determines the atom's chemical behavior and its ability to form bonds with other atoms. The arrangement of electrons in energy levels and orbitals dictates an element's reactivity and its place in chemical reactions.
The Proton's Defining Role: Atomic Number
The atomic number is the defining characteristic of an element. It's simply the number of protons found in the nucleus of an atom. This number is unique to each element; no two elements share the same atomic number. For example:
- Hydrogen (H): Atomic number 1 (1 proton)
- Helium (He): Atomic number 2 (2 protons)
- Oxygen (O): Atomic number 8 (8 protons)
- Gold (Au): Atomic number 79 (79 protons)
The periodic table, the iconic chart organizing elements, is arranged primarily by increasing atomic number. This arrangement reflects the fundamental role of protons in determining elemental identity. Moving across the table, we see a systematic increase in the number of protons, leading to a predictable change in the chemical and physical properties of the elements.
Isotopes: The Role of Neutrons in Variation
While protons define the element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. Isotopes have the same atomic number (same number of protons) but different mass numbers (total number of protons and neutrons).
For example, carbon (atomic number 6) has three naturally occurring isotopes:
- Carbon-12 (¹²C): 6 protons, 6 neutrons
- Carbon-13 (¹³C): 6 protons, 7 neutrons
- Carbon-14 (¹⁴C): 6 protons, 8 neutrons
Although these are all carbon atoms (same number of protons), their differing neutron counts lead to subtle variations in their mass and some nuclear properties. Carbon-14, for example, is radioactive, decaying over time, while Carbon-12 and Carbon-13 are stable. This highlights the importance of neutrons in influencing atomic stability and behavior, even though they don't determine the elemental identity.
Electrons and Chemical Properties
While protons define the element, electrons dictate its chemical behavior. Electrons occupy specific energy levels or shells surrounding the nucleus. The outermost shell, the valence shell, contains the valence electrons, which are directly involved in chemical bonding. The number of valence electrons largely determines an element's reactivity and how it interacts with other atoms.
Elements with similar numbers of valence electrons tend to exhibit similar chemical properties, a pattern reflected in the periodic table's arrangement into groups or families (columns). For instance, the alkali metals (Group 1) all have one valence electron, leading to their high reactivity. Noble gases (Group 18), on the other hand, have full valence shells, making them largely unreactive.
This connection between electrons and chemical properties is crucial in understanding chemical reactions and the formation of molecules. Electrons are exchanged or shared between atoms during chemical bonding, leading to the formation of compounds with distinct properties.
The Nucleus: The Heart of the Atom
The nucleus, containing both protons and neutrons, is the dense, positively charged core of the atom. It occupies a tiny fraction of the atom's overall volume but contains almost all of its mass. The strong nuclear force, a powerful fundamental force, binds the protons and neutrons together within the nucleus, overcoming the electrostatic repulsion between the positively charged protons.
The stability of the nucleus is a crucial factor in determining an atom's overall stability and whether it's radioactive. The ratio of protons to neutrons significantly impacts nuclear stability. Atoms with an unstable nucleus may undergo radioactive decay, emitting particles or energy to achieve a more stable configuration.
Beyond Protons: Isobars and Isotones
While protons are the ultimate determinant of an element's identity, it's helpful to consider other relationships between isotopes and related atomic species:
-
Isobars: Atoms with the same mass number (protons + neutrons) but different atomic numbers (different numbers of protons). Isobars represent different elements with the same total number of nucleons (protons and neutrons).
-
Isotones: Atoms with the same number of neutrons but different numbers of protons and, therefore, different atomic numbers. Isotones illustrate how variations in the number of protons, even with a constant neutron number, leads to different elements.
Conclusion: The Proton's Paramount Importance
In summary, the proton stands as the single subatomic particle definitively responsible for determining the identity of an atom. The number of protons, the atomic number, uniquely identifies an element and its position on the periodic table. While neutrons influence the atom's mass and stability, and electrons govern its chemical properties, it's the proton that provides the fundamental definition of an element's identity. The consistent and unwavering role of the proton in defining elemental identity underscores its central place in the world of atomic structure and the vast array of elements that make up our universe. Understanding the proton's defining role is fundamental to comprehending the behavior of matter at its most basic level.
Latest Posts
Latest Posts
-
What Is The Equivalent Fraction Of 3 8
Apr 02, 2025
-
Limit Of X As X Approaches 0
Apr 02, 2025
-
Lowest Common Multiple Of 28 And 42
Apr 02, 2025
-
Is Water A Pure Substance Or Mixture
Apr 02, 2025
-
What Is 40 Percent Of 1200
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Subatomic Particle Determines The Identity Of An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
