What Is The Conjugate Acid Of Hco3-
listenit
Apr 01, 2025 · 6 min read
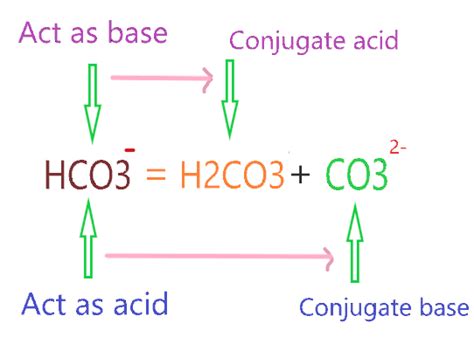
Table of Contents
What is the Conjugate Acid of HCO₃⁻? A Deep Dive into Acid-Base Chemistry
Understanding conjugate acid-base pairs is fundamental to grasping acid-base chemistry. This article delves into the concept of conjugate acids, specifically focusing on the conjugate acid of the bicarbonate ion, HCO₃⁻. We'll explore its formation, properties, and significance in various chemical and biological systems.
Understanding Conjugate Acid-Base Pairs
According to the Brønsted-Lowry theory of acids and bases, an acid is a substance that donates a proton (H⁺), while a base is a substance that accepts a proton. When an acid donates a proton, it forms its conjugate base, which is the species remaining after the proton is lost. Conversely, when a base accepts a proton, it forms its conjugate acid. These two species, the acid and its conjugate base (or the base and its conjugate acid), constitute a conjugate acid-base pair. They differ by only a single proton.
Key characteristics of conjugate acid-base pairs:
- They differ by one proton (H⁺): This is the defining feature. The conjugate acid has one more proton than the conjugate base.
- They are related through a reversible reaction: The acid's proton donation is typically a reversible process, meaning the conjugate base can accept a proton back to reform the original acid.
- Their strengths are inversely related: A strong acid has a weak conjugate base, and a weak acid has a relatively stronger conjugate base (and vice versa for bases and conjugate acids).
Identifying the Conjugate Acid of HCO₃⁻
The bicarbonate ion, HCO₃⁻, acts as a weak base. This means it can accept a proton. To find its conjugate acid, we simply need to add a proton (H⁺) to the bicarbonate ion:
HCO₃⁻ + H⁺ ⇌ H₂CO₃
The resulting species, H₂CO₃, is carbonic acid. Therefore, the conjugate acid of HCO₃⁻ is H₂CO₃ (carbonic acid).
Properties of Carbonic Acid (H₂CO₃)
Carbonic acid is a weak diprotic acid, meaning it can donate two protons. However, it's unstable in aqueous solution and readily decomposes into carbon dioxide (CO₂) and water (H₂O):
H₂CO₃ ⇌ CO₂ + H₂O
This decomposition is crucial in understanding the role of carbonic acid and the bicarbonate buffer system in biological systems. While the actual concentration of H₂CO₃ in solution is low due to its decomposition, the equilibrium between H₂CO₃, CO₂, and H₂O is essential for maintaining pH balance.
Acid Dissociation Constants (Ka)
The strength of an acid is quantified by its acid dissociation constant, Ka. For a diprotic acid like carbonic acid, there are two Ka values representing the stepwise dissociation of protons:
-
Ka₁ (first dissociation): This represents the dissociation of the first proton: H₂CO₃ ⇌ H⁺ + HCO₃⁻
-
Ka₂ (second dissociation): This represents the dissociation of the second proton: HCO₃⁻ ⇌ H⁺ + CO₃²⁻
The Ka values for carbonic acid are relatively small, reflecting its weak acidity. The low Ka values indicate that carbonic acid does not readily donate its protons.
Significance of Carbonic Acid and Bicarbonate in Biological Systems
The carbonic acid-bicarbonate buffer system plays a vital role in maintaining the pH of blood and other bodily fluids within a narrow range (around 7.4). This buffer system works by resisting changes in pH when small amounts of acid or base are added.
How the buffer system works:
-
Addition of acid (H⁺): The added H⁺ reacts with the bicarbonate ion (HCO₃⁻) to form carbonic acid (H₂CO₃), which then decomposes into CO₂ and H₂O. This minimizes the increase in H⁺ concentration and thus prevents a significant drop in pH.
-
Addition of base (OH⁻): The added OH⁻ reacts with carbonic acid (H₂CO₃) to form bicarbonate (HCO₃⁻) and water (H₂O). This minimizes the decrease in H⁺ concentration and thus prevents a significant rise in pH.
This buffer system's effectiveness relies on the equilibrium between carbonic acid, bicarbonate, and carbon dioxide, ensuring that the pH remains relatively stable despite fluctuations in acid or base levels. The respiratory system and kidneys work in conjunction with this buffer system to regulate blood pH. The lungs control the CO₂ level, influencing the equilibrium and ultimately affecting the H⁺ concentration. The kidneys regulate the excretion of bicarbonate and other ions to further fine-tune the pH.
HCO₃⁻ in Other Chemical Contexts
Beyond its biological significance, the bicarbonate ion and its conjugate acid have applications in various chemical contexts:
- Industrial processes: Bicarbonate is used in various industrial processes, including food production (as a leavening agent), pharmaceuticals, and fire extinguishers.
- Water treatment: Bicarbonate is a natural component of many water sources and plays a role in water hardness. Understanding its acid-base chemistry is important in water treatment processes.
- Chemical analysis: Titration methods utilize acid-base reactions, including those involving bicarbonate and carbonic acid, for quantitative analysis of various substances.
Exploring Further: Related Concepts and Applications
This section expands on related concepts and applications to provide a more comprehensive understanding of the bicarbonate ion and its conjugate acid:
Amphoteric Nature of HCO₃⁻
The bicarbonate ion (HCO₃⁻) exhibits amphoteric behavior, meaning it can act as both an acid and a base. As demonstrated earlier, it can act as a base by accepting a proton to form carbonic acid (H₂CO₃). However, it can also act as a weak acid by donating a proton to form the carbonate ion (CO₃²⁻):
HCO₃⁻ ⇌ H⁺ + CO₃²⁻
This dual nature is crucial to its role in buffer systems, enabling it to neutralize both acids and bases.
The Carbonate Buffer System
While the carbonic acid-bicarbonate buffer system is prominent in biological systems, it's essential to note that the carbonate system (involving CO₃²⁻, HCO₃⁻, and H₂CO₃) plays a role in various natural environments, such as oceans and lakes, influencing their pH and overall chemistry.
Acid-Base Titrations involving HCO₃⁻
Titration is a quantitative analytical technique used to determine the concentration of an unknown solution. Acid-base titrations frequently involve bicarbonate and carbonic acid. For example, the titration of a bicarbonate solution with a strong acid, such as HCl, allows for the precise determination of the bicarbonate concentration based on the volume of acid required to reach the equivalence point.
The Role of pH in Chemical Reactions and Processes
The pH of a solution significantly influences the course and rate of many chemical reactions. Understanding the acid-base properties of species like HCO₃⁻ and H₂CO₃ is crucial for predicting and controlling reaction outcomes in various chemical and industrial processes.
Conclusion: A Comprehensive Understanding of HCO₃⁻ and its Conjugate Acid
The bicarbonate ion (HCO₃⁻) and its conjugate acid, carbonic acid (H₂CO₃), are integral components of numerous chemical and biological systems. Understanding their properties, their roles in acid-base equilibria, and their significance in maintaining pH homeostasis is crucial for various scientific disciplines. From biological systems to industrial applications, the chemistry of these species underpins numerous processes and phenomena. This comprehensive exploration sheds light on the importance of conjugate acid-base pairs and their influence on the world around us. The profound impact of this seemingly simple acid-base relationship underscores the intricate and interconnected nature of chemistry.
Latest Posts
Latest Posts
-
Lowest Common Multiple Of 28 And 42
Apr 02, 2025
-
Is Water A Pure Substance Or Mixture
Apr 02, 2025
-
What Is 40 Percent Of 1200
Apr 02, 2025
-
21 Is What Percent Of 25
Apr 02, 2025
-
An Organism That Eats Only Plants
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Acid Of Hco3- . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
