What Are The Monomers And Polymers
listenit
Mar 17, 2025 · 7 min read
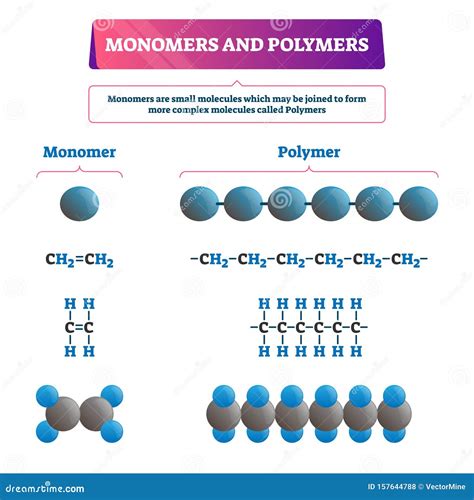
Table of Contents
What are Monomers and Polymers? A Deep Dive into the Building Blocks of Matter
The world around us is built from a vast array of materials, from the sturdy wood of a tree to the flexible plastic of a water bottle. At the heart of this diversity lies the concept of monomers and polymers. Understanding these fundamental building blocks is key to grasping the properties and applications of countless substances. This comprehensive guide will explore the fascinating world of monomers and polymers, covering their definitions, types, properties, and widespread applications.
What are Monomers?
A monomer is a small, simple molecule that can react with other monomers to form a larger molecule known as a polymer. Think of monomers as individual Lego bricks – they are relatively small and simple units, but when combined, they create complex structures with unique properties. The term "monomer" is derived from the Greek words "monos" (single) and "meros" (part). Monomers are the fundamental repeating units that make up polymers. Their chemical structure dictates the properties of the resulting polymer.
Types of Monomers: A Diverse Family
Monomers come in a vast array of shapes, sizes, and chemical compositions. Some common types include:
-
Amino acids: These are the monomers that build proteins. Each amino acid has a unique side chain, resulting in the vast diversity of proteins found in living organisms. Examples include glycine, alanine, and valine. The sequence of amino acids determines the protein's three-dimensional structure and function.
-
Nucleotides: These are the monomers that make up nucleic acids like DNA and RNA. Each nucleotide consists of a sugar molecule (ribose or deoxyribose), a phosphate group, and a nitrogenous base (adenine, guanine, cytosine, thymine, or uracil). The specific sequence of nucleotides in DNA and RNA encodes genetic information.
-
Monosaccharides: These are simple sugars, the monomers of carbohydrates. Glucose, fructose, and galactose are examples of monosaccharides. They can link together to form disaccharides (like sucrose) and polysaccharides (like starch and cellulose).
-
Fatty acids: These are the monomers of lipids, which are essential components of cell membranes. Fatty acids are long chains of carbon atoms with a carboxyl group at one end. They can be saturated (no double bonds between carbon atoms) or unsaturated (containing one or more double bonds).
-
Isoprene: This five-carbon molecule is the monomer of many natural and synthetic polymers, including rubber and various terpenes. Isoprene's ability to form various polymer chains contributes to the vast diversity of isoprene-based materials.
What are Polymers?
A polymer is a large molecule composed of many repeating smaller units called monomers. The process of combining monomers to form polymers is called polymerization. Polymers are essentially long chains of monomers linked together by covalent bonds. The length and arrangement of these chains greatly influence the properties of the polymer. The term "polymer" is derived from the Greek words "poly" (many) and "meros" (part).
Types of Polymers: A Spectrum of Properties
Polymers exhibit a vast range of properties depending on the type of monomers they are made from and how those monomers are arranged. They can be categorized in several ways:
-
Natural Polymers: These are polymers found in nature, such as proteins, carbohydrates, nucleic acids, and natural rubber. They play crucial roles in biological systems.
-
Synthetic Polymers: These are polymers produced artificially through chemical processes. Examples include plastics (like polyethylene, polypropylene, and polystyrene), synthetic fibers (like nylon and polyester), and synthetic rubbers. Synthetic polymers have revolutionized various industries, from packaging to textiles to construction.
-
Addition Polymers: These polymers are formed by the addition of monomers without the loss of any atoms. The monomers directly link together to form a long chain. Polyethylene, formed from ethylene monomers, is a prime example.
-
Condensation Polymers: These polymers are formed when monomers join together with the loss of a small molecule, such as water. Nylon and polyester are examples of condensation polymers. The elimination of a small molecule during polymerization is a defining characteristic of this type.
-
Homopolymers: These consist of only one type of monomer. Polyethylene, for instance, is a homopolymer of ethylene. Their properties are relatively straightforward, often determined by the properties of the individual monomers.
-
Copolymers: These are polymers made from two or more different types of monomers. The properties of copolymers can be tailored by varying the types and proportions of monomers used. This allows for a wide range of material properties to be achieved.
Properties of Polymers: A Tailored Approach
The remarkable versatility of polymers stems from the wide range of properties they can exhibit. These properties are significantly influenced by:
-
Chain Length: Longer polymer chains generally lead to increased strength, higher melting points, and greater viscosity.
-
Chain Branching: Branched polymer chains tend to be less dense and more flexible than linear chains.
-
Cross-linking: Cross-linking involves the formation of covalent bonds between different polymer chains. This increases strength, rigidity, and thermal stability.
-
Monomer Type: The chemical nature of the monomers significantly affects the overall properties of the polymer. For example, polymers with polar monomers tend to be more hydrophilic (water-loving) than those with nonpolar monomers.
-
Crystallinity: The degree to which the polymer chains are arranged in an ordered crystalline structure influences properties like strength, transparency, and melting point. Highly crystalline polymers are generally stronger and more rigid.
Applications of Polymers: Shaping Our World
Polymers have found countless applications across diverse industries, impacting nearly every aspect of modern life:
-
Packaging: Plastics like polyethylene and polypropylene are extensively used for packaging food, beverages, and other products. Their flexibility, low cost, and barrier properties make them ideal for this purpose.
-
Textiles: Synthetic polymers like nylon, polyester, and acrylic are used to produce a wide variety of fabrics for clothing, carpets, and other textiles. Their durability, washability, and versatility have made them indispensable in the textile industry.
-
Construction: Polymers are used in construction materials such as pipes, insulation, and coatings. Their strength, durability, and resistance to corrosion make them suitable for various applications in the construction sector.
-
Automotive: Polymers are used extensively in the automotive industry for components such as dashboards, bumpers, and interior trim. Their lightweight nature, formability, and impact resistance make them ideal for automotive applications.
-
Medicine: Polymers are used in medical devices such as implants, drug delivery systems, and diagnostic tools. Biocompatible polymers are crucial in biomedical applications due to their ability to interact with living tissues without causing adverse reactions.
-
Electronics: Polymers are used in electronic components such as insulators, capacitors, and printed circuit boards. Their electrical insulating properties and ability to be easily processed make them suitable for electronics applications.
The Future of Polymers: Innovation and Sustainability
The field of polymer science continues to evolve rapidly, with ongoing research focusing on the development of:
-
Biodegradable polymers: These polymers can be broken down by microorganisms, reducing environmental pollution associated with plastic waste. This area of research is crucial in addressing concerns about plastic pollution.
-
Bio-based polymers: These are polymers derived from renewable biomass sources such as plants, reducing reliance on fossil fuels. The development of bio-based polymers contributes to a more sustainable approach to materials production.
-
Smart polymers: These polymers can respond to changes in their environment, such as temperature, pH, or light. Their responsiveness makes them ideal for advanced applications in areas like drug delivery and sensors.
-
High-performance polymers: These polymers exhibit exceptional properties such as high strength, high temperature resistance, and chemical inertness. They find use in demanding applications such as aerospace and high-performance sports equipment.
Conclusion: Monomers and Polymers – The Foundation of Material Science
Monomers and polymers are fundamental building blocks of countless materials that shape our world. Their diverse properties and wide range of applications highlight their importance in various industries. The ongoing advancements in polymer science promise even more innovative and sustainable materials in the years to come. Understanding the intricacies of monomers and polymers is essential for anyone seeking to comprehend the materials that surround us and contribute to future material innovations. The versatility and adaptability of these fundamental units are truly remarkable, continuing to drive technological progress and address global challenges.
Latest Posts
Latest Posts
-
What Is Difference Between Atom And Ion
Mar 17, 2025
-
What Gas Is Used In The Process
Mar 17, 2025
-
Which Is The Electron Configuration For Bromine
Mar 17, 2025
-
How Many Protons Does Mercury Have
Mar 17, 2025
-
What Is An Example Of Abiotic Factor
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Are The Monomers And Polymers . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
