What Is Difference Between Atom And Ion
listenit
Mar 17, 2025 · 6 min read
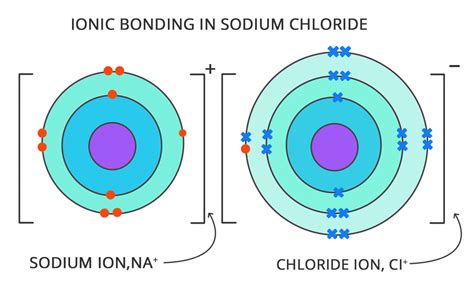
Table of Contents
What's the Difference Between an Atom and an Ion? A Deep Dive into Atomic Structure and Charge
Understanding the fundamental building blocks of matter is crucial for grasping the complexities of chemistry and physics. At the heart of this understanding lies the distinction between atoms and ions. While seemingly subtle, this difference profoundly impacts the properties and behaviors of substances. This article will explore the core differences between atoms and ions, delving into their structures, charges, and the processes that transform one into the other. We'll also examine the role they play in various chemical reactions and phenomena.
Atoms: The Fundamental Units of Matter
Atoms are the basic units of all chemical elements. They are incredibly small, typically measuring only about 100 picometers (1 picometer = 1 trillionth of a meter) in diameter. Despite their minuscule size, atoms are complex structures containing three fundamental subatomic particles:
Subatomic Particles: Protons, Neutrons, and Electrons
-
Protons: Positively charged particles located within the atom's nucleus (the central core). The number of protons determines the atomic number of an element and uniquely identifies it on the periodic table. For example, all hydrogen atoms have one proton, all carbon atoms have six, and so on. Protons contribute significantly to the atom's mass.
-
Neutrons: Neutrally charged particles also found in the nucleus. Neutrons, along with protons, contribute to the atom's mass. The number of neutrons in an atom of a particular element can vary, leading to isotopes. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons.
-
Electrons: Negatively charged particles that orbit the nucleus in specific energy levels or shells. Electrons are significantly lighter than protons and neutrons. The number of electrons in a neutral atom is equal to the number of protons. These electrons are crucial for chemical bonding and determining the chemical properties of an element.
Atomic Number and Mass Number
The atomic number represents the number of protons in an atom's nucleus. This number uniquely identifies each element. The mass number represents the total number of protons and neutrons in the nucleus. Since the mass of electrons is negligible compared to protons and neutrons, the mass number is a good approximation of the atom's mass.
Ions: Charged Atoms
An ion is an atom or molecule that has gained or lost one or more electrons, resulting in a net electrical charge. Unlike neutral atoms, where the number of protons and electrons are equal, ions possess an imbalance in these charges.
Cation: Positively Charged Ions
A cation is a positively charged ion. This occurs when an atom loses one or more electrons. The loss of negatively charged electrons leaves the atom with more positively charged protons, resulting in a net positive charge. For example, a sodium atom (Na) can lose one electron to become a sodium cation (Na⁺).
Anion: Negatively Charged Ions
An anion is a negatively charged ion. This occurs when an atom gains one or more electrons. The gain of negatively charged electrons results in an excess of negative charge compared to the number of protons, giving the atom a net negative charge. For example, a chlorine atom (Cl) can gain one electron to become a chloride anion (Cl⁻).
The Process of Ion Formation: Ionization
The process of forming ions is called ionization. Ionization can occur through several mechanisms, including:
-
Electron transfer: This is the most common mechanism. It involves the transfer of electrons from one atom to another. Atoms with low ionization energies (easily lose electrons) readily form cations, while atoms with high electron affinities (easily gain electrons) readily form anions.
-
Electromagnetic radiation: High-energy electromagnetic radiation, such as X-rays or gamma rays, can knock electrons out of atoms, creating cations and free electrons.
-
Collision with high-energy particles: Collisions with high-energy particles, such as in radioactive decay or particle accelerators, can also ionize atoms.
Key Differences Summarized: Atoms vs. Ions
| Feature | Atom | Ion |
|---|---|---|
| Charge | Neutral (no net charge) | Positive (cation) or negative (anion) |
| Electrons | Number of electrons equals protons | Number of electrons differs from protons |
| Stability | Generally stable | Can be more or less stable than parent atom |
| Formation | Fundamental building block of matter | Formed through ionization |
| Chemical Behavior | Determines by its outer electrons | Often highly reactive |
The Role of Ions in Chemical Reactions and Phenomena
Ions play a vital role in a wide range of chemical reactions and phenomena, including:
-
Ionic bonding: Ions with opposite charges attract each other, forming ionic bonds. These bonds are strong electrostatic forces that hold ions together in ionic compounds, such as sodium chloride (NaCl), commonly known as table salt.
-
Electrolyte solutions: When ionic compounds dissolve in water, they dissociate into their constituent ions, creating electrolyte solutions. These solutions conduct electricity because the ions are free to move and carry charge. Electrolyte solutions are crucial in biological systems, nerve impulse transmission, and many industrial processes.
-
Acid-base reactions: Many acid-base reactions involve the transfer of protons (H⁺ ions). Acids donate protons, while bases accept protons.
-
Redox reactions: Oxidation-reduction (redox) reactions involve the transfer of electrons. Oxidation is the loss of electrons (forming cations), while reduction is the gain of electrons (forming anions). Redox reactions are essential for energy production in living organisms and many industrial processes.
Real-World Examples of Atoms and Ions
Understanding the difference between atoms and ions becomes clear when considering real-world examples:
-
Sodium (Na): A sodium atom is a neutral entity with 11 protons and 11 electrons. However, a sodium ion (Na⁺) has lost one electron, resulting in a positive charge and significantly different chemical reactivity. It readily reacts with chlorine to form sodium chloride.
-
Chlorine (Cl): A chlorine atom is a neutral species with 17 protons and 17 electrons. A chlorine ion (Cl⁻), also called a chloride ion, has gained one electron, becoming negatively charged. This negative charge allows it to form strong ionic bonds with positively charged ions such as sodium.
-
Calcium (Ca): Calcium atoms are relatively unreactive. However, calcium ions (Ca²⁺) are crucial for various biological processes, such as bone formation and muscle contraction. They are highly reactive and readily form ionic bonds.
Conclusion: A Fundamental Distinction
The difference between atoms and ions, while seemingly subtle, is fundamental to understanding the behavior of matter. Atoms are the basic building blocks of elements, characterized by their neutral charge and equal numbers of protons and electrons. Ions, formed through the gain or loss of electrons, carry a net electrical charge and exhibit distinct chemical properties compared to their parent atoms. This distinction has profound implications for chemical bonding, reactivity, and a myriad of phenomena across various scientific disciplines. A thorough understanding of atoms and ions is essential for anyone pursuing a deeper understanding of chemistry, physics, and related fields. Further research into the specific ionization processes and the diverse behavior of different ions is encouraged for a more comprehensive understanding.
Latest Posts
Latest Posts
-
Which Of The Following Is Present In A Prokaryotic Cell
Mar 17, 2025
-
How Are Monomers And Polymers Related
Mar 17, 2025
-
What Is 8 9 As A Decimal
Mar 17, 2025
-
Least Common Multiple Of 2 And 8
Mar 17, 2025
-
Evaluate The Integral By Interpreting It In Terms Of Areas
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is Difference Between Atom And Ion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
