How Are Monomers And Polymers Related
listenit
Mar 17, 2025 · 6 min read
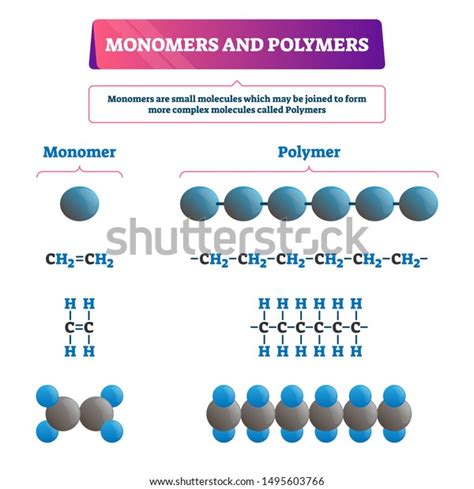
Table of Contents
- How Are Monomers And Polymers Related
- Table of Contents
- How are Monomers and Polymers Related? A Deep Dive into Macromolecular Structure and Function
- What are Monomers? The Building Blocks of Polymers
- Examples of Monomers: A Diverse Chemical Landscape
- What are Polymers? Macromolecules with Unique Properties
- Properties of Polymers: A Spectrum of Characteristics
- The Polymerization Process: From Monomers to Polymers
- 1. Addition Polymerization: Chain Reaction Magic
- 2. Condensation Polymerization: Water as a Byproduct
- The Relationship in Detail: A Closer Look at Structure and Function
- The Importance of Monomers and Polymers in Our Daily Lives
- Conclusion: A Symbiotic Relationship Shaping Our World
- Latest Posts
- Latest Posts
- Related Post
How are Monomers and Polymers Related? A Deep Dive into Macromolecular Structure and Function
Understanding the relationship between monomers and polymers is fundamental to grasping the essence of polymer chemistry and its vast applications across various scientific disciplines and industries. From the synthetic fibers in our clothing to the complex biomolecules sustaining life, the connection between these building blocks shapes the world around us. This article will delve into this relationship, exploring the intricacies of monomeric units, polymerization processes, and the diverse properties of resulting polymeric materials.
What are Monomers? The Building Blocks of Polymers
Monomers are small, relatively simple molecules that serve as the fundamental units for the construction of larger macromolecules called polymers. Think of them as the individual bricks used to build a magnificent wall. These monomers possess reactive functional groups, chemical sites capable of forming strong covalent bonds with other monomers. These reactive sites are crucial for the polymerization process, the chain reaction that binds monomers together. The chemical nature of the functional group significantly influences the type of polymerization reaction and the properties of the resulting polymer.
Examples of Monomers: A Diverse Chemical Landscape
The world of monomers is incredibly diverse, encompassing a wide array of chemical structures and functional groups. Examples include:
-
Ethylene (CH₂=CH₂): A simple alkene, ethylene is the monomer for polyethylene (PE), a ubiquitous plastic used in countless applications. Its double bond allows for addition polymerization.
-
Styrene (C₈H₈): This aromatic monomer is the building block of polystyrene (PS), a common plastic known for its versatility and transparency. Again, the double bond is key to its polymerization.
-
Vinyl Chloride (CH₂=CHCl): The monomer for polyvinyl chloride (PVC), a rigid and durable plastic used in pipes, flooring, and many other products. The chlorine atom introduces unique properties into the polymer chain.
-
Amino acids: These are the monomers of proteins, the workhorses of biological systems. Each amino acid possesses an amino (-NH₂) group and a carboxyl (-COOH) group, which participate in peptide bond formation during protein synthesis. The diversity of amino acids (20 standard ones) leads to the incredible diversity of protein structures and functions.
-
Nucleotides: These are the monomers of nucleic acids like DNA and RNA, the carriers of genetic information. Each nucleotide consists of a sugar (ribose or deoxyribose), a phosphate group, and a nitrogenous base (adenine, guanine, cytosine, thymine, or uracil). The specific sequence of nucleotides determines the genetic code.
-
Glucose (C₆H₁₂O₆): This simple sugar is a monomer for various polysaccharides like cellulose (plant cell walls) and starch (energy storage in plants). The linkage of glucose units through glycosidic bonds creates these complex carbohydrates.
What are Polymers? Macromolecules with Unique Properties
Polymers are macromolecules—giant molecules—formed by the covalent bonding of numerous monomeric units. This repetitive arrangement of monomers gives polymers their unique properties, which often differ significantly from the properties of their constituent monomers. The length of the polymer chain (degree of polymerization), the arrangement of monomers within the chain (tacticity), and the presence of side groups all contribute to the final characteristics of the polymer.
Properties of Polymers: A Spectrum of Characteristics
The properties of polymers are highly variable and depend on several factors, including:
-
Molecular Weight: Higher molecular weight generally leads to increased strength, toughness, and melting point.
-
Chain Structure: Linear, branched, or cross-linked structures influence flexibility, elasticity, and crystallinity.
-
Intermolecular Forces: Forces between polymer chains (van der Waals forces, hydrogen bonds) affect the polymer's melting point, solubility, and mechanical properties.
-
Crystallinity: The degree of crystallinity (ordered arrangement of polymer chains) impacts strength, stiffness, and transparency.
-
Chemical Composition: The type of monomers and their arrangement dictate the chemical resistance, thermal stability, and other properties.
These properties are carefully tailored by controlling the polymerization process and modifying the monomer structure.
The Polymerization Process: From Monomers to Polymers
Polymerization is the process of chemically bonding monomers together to form a polymer chain. There are two main types of polymerization:
1. Addition Polymerization: Chain Reaction Magic
Addition polymerization involves the sequential addition of monomers to a growing chain without the loss of any atoms. It typically occurs with unsaturated monomers containing double or triple bonds. The reaction initiates with a free radical, ion, or a catalyst, which then attacks the double bond, creating a reactive site that adds another monomer, and so on, until the chain is terminated. Examples include the formation of polyethylene, polystyrene, and PVC.
2. Condensation Polymerization: Water as a Byproduct
Condensation polymerization involves the joining of monomers with the simultaneous removal of a small molecule, often water. This process typically occurs between monomers with two or more functional groups capable of reacting with each other. The resulting polymer chain is formed by the formation of an ester, amide, or other linkage. Examples include the formation of nylon (from diamines and diacids) and polyester (from diols and diacids).
The Relationship in Detail: A Closer Look at Structure and Function
The relationship between monomers and polymers is one of structure determining function. The chemical structure of the monomer dictates the type of polymerization, the properties of the resulting polymer, and ultimately, its applications. For example:
-
Polyethylene (PE): The simple structure of ethylene (CH₂=CH₂) leads to a flexible, relatively low-strength polymer suitable for plastic bags and films.
-
Polytetrafluoroethylene (PTFE, Teflon): The introduction of fluorine atoms in the monomer (tetrafluoroethylene) results in a highly non-stick, chemically inert polymer ideal for cookware coatings.
-
Polypropylene (PP): The addition of a methyl group to ethylene (propylene) leads to a stronger, more crystalline polymer with various applications, including packaging and fibers.
This principle applies equally to biological polymers. The sequence and arrangement of amino acids in a protein determine its three-dimensional structure and function. Similarly, the sequence of nucleotides in DNA dictates the genetic code and the synthesis of proteins.
The Importance of Monomers and Polymers in Our Daily Lives
The impact of monomers and polymers on our daily lives is immense and pervasive. They are essential components of countless products, including:
-
Packaging: Plastics like polyethylene, polypropylene, and polystyrene are extensively used in food packaging, protecting and preserving our food.
-
Clothing: Synthetic fibers like nylon, polyester, and acrylic are widely used in clothing, offering durability, comfort, and various properties.
-
Construction: Polymers are used in paints, adhesives, insulation materials, and other building components.
-
Transportation: Polymers are essential in car parts, tires, and aircraft components.
-
Medicine: Biocompatible polymers are used in drug delivery systems, implants, and medical devices.
-
Electronics: Polymers are used in electronic components and insulating materials.
Conclusion: A Symbiotic Relationship Shaping Our World
The relationship between monomers and polymers is a fundamental concept in chemistry and materials science. The precise chemical structure of the monomer dictates the properties and functions of the resulting polymer. This symbiotic relationship allows for the design and synthesis of polymers with tailored properties for a wide array of applications, profoundly impacting our daily lives. From the mundane to the extraordinary, monomers and polymers underpin technological advancements and contribute to the complexity and richness of our world. Further research into novel monomers and polymerization techniques promises even more innovative materials in the future.
Latest Posts
Latest Posts
-
On The Trip From Detroit To Columbus
Mar 18, 2025
-
Is The Shoulder Distal To The Elbow
Mar 18, 2025
-
What Is The Percentage Of 1 12
Mar 18, 2025
-
Product Of Nacl Hn03 And Na2co3
Mar 18, 2025
-
What Is 0 01 As A Fraction
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Are Monomers And Polymers Related . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
