How Many Protons Does Mercury Have
listenit
Mar 17, 2025 · 6 min read
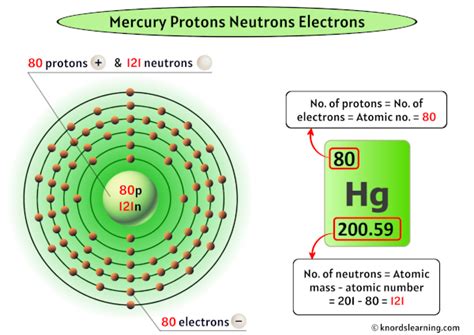
Table of Contents
How Many Protons Does Mercury Have? Unraveling the Atomic Structure of Mercury
Mercury, a fascinating element known for its unique properties and historical significance, holds a special place in the periodic table. Understanding its atomic structure, particularly the number of protons it possesses, is key to comprehending its behavior and characteristics. This in-depth exploration will delve into the atomic makeup of mercury, explaining not only the number of protons but also its implications for its physical and chemical properties, its applications, and its impact on the environment and human health.
Understanding Atomic Structure: The Foundation of Mercury's Identity
Before we pinpoint the exact number of protons in a mercury atom, it's essential to grasp the fundamental concepts of atomic structure. Atoms, the basic building blocks of matter, are composed of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's atomic number and its identity. No two elements have the same number of protons.
- Neutrons: Neutrally charged particles also located in the nucleus. The number of neutrons can vary within an element, leading to different isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons usually equals the number of protons in a neutral atom.
The arrangement of these particles dictates an atom's properties and how it interacts with other atoms. The positive charge of the protons in the nucleus is balanced by the negative charge of the electrons, resulting in a neutral atom.
Mercury's Atomic Number: The Key to its Proton Count
The atomic number of an element is the defining characteristic that determines its identity. It represents the number of protons found in the nucleus of an atom of that element. For mercury (Hg), the atomic number is 80. This crucial piece of information immediately tells us that every mercury atom contains 80 protons.
This seemingly simple number holds profound implications. The 80 protons in the nucleus determine the element's place on the periodic table, its chemical reactivity, its bonding behavior, and its overall physical properties.
Mercury's Position on the Periodic Table: A Reflection of its Proton Count
Mercury's position in the periodic table, specifically in Group 12 and Period 6, is a direct consequence of its 80 protons. The periodic table is organized based on atomic number, with elements arranged in order of increasing proton count. This arrangement highlights trends and patterns in elemental properties, allowing chemists to predict the behavior of different elements based on their position. Mercury's placement reflects its unique properties, such as its liquid state at room temperature, its high density, and its relatively low reactivity compared to other transition metals.
Isotopes of Mercury: Variations in Neutron Count
While the number of protons remains constant at 80 for all mercury atoms, the number of neutrons can vary. These variations create different isotopes of mercury. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. This means they have the same atomic number but different mass numbers (the sum of protons and neutrons).
Mercury has several naturally occurring isotopes, including:
- Mercury-196 (¹⁹⁶Hg): Contains 80 protons and 116 neutrons.
- Mercury-198 (¹⁹⁸Hg): Contains 80 protons and 118 neutrons. This is the most abundant isotope.
- Mercury-199 (¹⁹⁹Hg): Contains 80 protons and 119 neutrons.
- Mercury-200 (²⁰⁰Hg): Contains 80 protons and 120 neutrons.
- Mercury-201 (²⁰¹Hg): Contains 80 protons and 121 neutrons.
- Mercury-202 (²⁰²Hg): Contains 80 protons and 122 neutrons.
- Mercury-204 (²⁰⁴Hg): Contains 80 protons and 124 neutrons.
These different isotopes have slightly varying masses and properties, although their chemical behavior remains largely the same. The relative abundance of each isotope contributes to the average atomic mass of mercury, which is approximately 200.59 amu (atomic mass units).
The Significance of 80 Protons: Impact on Mercury's Properties
The presence of 80 protons in the mercury atom is directly responsible for its unique properties:
- Liquid State at Room Temperature: Mercury's unusual liquid state at room temperature is due to the weak metallic bonding between its atoms. This weak bonding is influenced by the electronic configuration arising from the 80 protons. The relatively weak interatomic forces allow the mercury atoms to move freely, resulting in its liquid form.
- High Density: Mercury's high density compared to other metals is a consequence of its high atomic mass and the tightly packed arrangement of its atoms. The 80 protons contribute significantly to this high atomic mass.
- Low Reactivity (Compared to other metals): Mercury's relatively low reactivity is linked to its electronic configuration and the stability of its filled electron shells. The 80 protons dictate this electronic configuration, making it less prone to readily participate in chemical reactions compared to other transition metals.
- Toxicity: The properties that make mercury unique also contribute to its toxicity. Its ability to form various compounds and its tendency to accumulate in living organisms are factors that pose significant health risks.
Applications of Mercury: Leveraging its Unique Properties
Despite its toxicity, mercury has historically been used in a wide range of applications, leveraging its unique physical and chemical properties. However, due to growing concerns about its environmental and health impacts, many of these applications are being phased out. Some past and present applications include:
- Thermometers and Barometers: Mercury's uniform thermal expansion and high density made it ideal for these instruments.
- Electrical Switches and Relays: Mercury's high electrical conductivity and liquid state were utilized in switches and relays.
- Dental Amalgam: Mercury was historically a key component of dental fillings.
- Fluorescent Lamps: Mercury vapor is used in fluorescent lamps to produce ultraviolet light.
- Industrial Processes: Mercury has been used in various industrial processes, including the production of chlorine and caustic soda.
Environmental and Health Concerns: The Dark Side of Mercury
The toxicity of mercury is a significant concern. Mercury can enter the environment through various sources, including industrial emissions, mining activities, and the disposal of mercury-containing products. Once released, mercury can undergo bioaccumulation in the food chain, posing severe health risks to humans and wildlife. Symptoms of mercury poisoning can range from neurological disorders and kidney damage to developmental problems in children. The widespread awareness of these dangers has led to significant efforts to reduce mercury emissions and eliminate its use in many applications.
Conclusion: The Importance of Understanding Mercury's Atomic Structure
The seemingly simple question, "How many protons does mercury have?" opens a gateway to a deeper understanding of this fascinating element. The answer – 80 – is the foundation upon which its unique properties, applications, and environmental impacts are built. The number of protons not only dictates mercury's position on the periodic table but also governs its behavior in chemical reactions and its interactions with the environment. Understanding the atomic structure of mercury is crucial for appreciating its significance in various fields, managing its environmental impact, and protecting human health. While its uses are decreasing due to its toxicity, the fundamental knowledge of its atomic structure remains essential for scientific advancement and responsible stewardship of our planet.
Latest Posts
Latest Posts
-
Which Of The Following Is Present In A Prokaryotic Cell
Mar 17, 2025
-
How Are Monomers And Polymers Related
Mar 17, 2025
-
What Is 8 9 As A Decimal
Mar 17, 2025
-
Least Common Multiple Of 2 And 8
Mar 17, 2025
-
Evaluate The Integral By Interpreting It In Terms Of Areas
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Does Mercury Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
