What Are 3 Subatomic Particles And Their Charges
listenit
Apr 01, 2025 · 7 min read
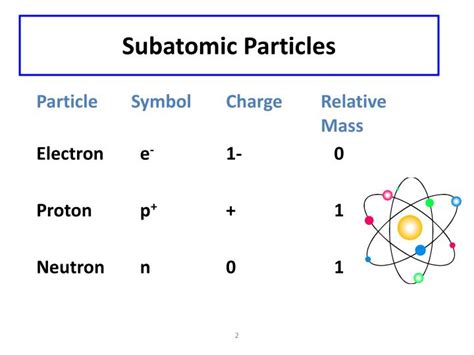
Table of Contents
What are the 3 Subatomic Particles and Their Charges? Delving into the Heart of Matter
The world around us, from the smallest grain of sand to the largest mountain, is made up of matter. But what constitutes matter at its most fundamental level? The answer lies in the realm of subatomic particles, the tiny building blocks that form the atoms that make up everything we see and interact with. While there are many subatomic particles discovered in particle physics, three stand out as the fundamental constituents of ordinary matter: protons, neutrons, and electrons. Understanding their properties, particularly their electric charges, is crucial to grasping the nature of matter and its interactions.
The Proton: The Positively Charged Core
The proton is a subatomic particle found in the nucleus, the dense central core of an atom. It carries a single unit of positive electric charge, conventionally denoted as +1. This positive charge is fundamental to the proton's identity and its role in atomic structure. The number of protons in an atom's nucleus defines the element; for example, an atom with one proton is hydrogen, while an atom with six protons is carbon.
Mass and Size of a Proton
Protons are significantly more massive than electrons. Their mass is approximately 1,836 times that of an electron. While the precise size of a proton remains a subject of ongoing research in physics, it's incredibly small, with a radius on the order of 0.84 femtometers (a femtometer is one quadrillionth of a meter!). Their small size, combined with their positive charge, dictates their pivotal role in the atom's overall charge and interactions.
The Strong Nuclear Force: Holding the Nucleus Together
The positive charges of multiple protons within the nucleus would naturally cause them to repel each other due to the electromagnetic force. However, the nucleus remains stable due to another fundamental force known as the strong nuclear force. This force is much stronger than the electromagnetic force at short distances and overcomes the electrostatic repulsion between protons, holding the nucleus together. The strength of the strong nuclear force is intimately connected to the number of protons and neutrons in the nucleus, and it plays a critical role in the stability of different isotopes of elements.
The Neutron: The Neutral Nuclear Partner
The neutron, also residing in the atom's nucleus, is a subatomic particle with no net electric charge. Its charge is neutral, or 0. Despite its lack of charge, the neutron plays a crucial role in the atom's stability and properties. The number of neutrons in an atom's nucleus, along with the number of protons, determines the isotope of the element. Isotopes are variants of an element with the same number of protons but differing numbers of neutrons.
Mass and Size of a Neutron
Neutrons have a mass slightly larger than that of protons, and like protons, their size is incredibly small, on the order of a femtometer. The slight difference in mass between protons and neutrons contributes to the complexities of nuclear physics and the stability of different isotopes.
The Role of Neutrons in Nuclear Stability
The presence of neutrons is essential for the stability of many atomic nuclei. Neutrons help to moderate the repulsive forces between protons within the nucleus, allowing for the existence of heavier elements with many protons. Without the neutrons, the electromagnetic repulsion would overwhelm the strong nuclear force, leading to nuclear instability and radioactive decay. The neutron-to-proton ratio in a nucleus is a critical factor in determining its stability.
Neutron Decay
Free neutrons, those not bound within an atomic nucleus, are unstable and decay with a half-life of about 10 minutes. This decay process involves the transformation of a neutron into a proton, an electron, and an antineutrino. This decay highlights the intricate interconnections between different subatomic particles and the forces that govern their interactions.
The Electron: The Negatively Charged Orbiting Particle
The electron is a subatomic particle found orbiting the nucleus of an atom. It carries a single unit of negative electric charge, conventionally denoted as -1. This negative charge is precisely equal in magnitude but opposite in sign to the positive charge of a proton. This balance of positive and negative charges within an atom is what typically gives it a neutral overall charge.
Mass and Size of an Electron
Electrons are significantly lighter than protons and neutrons, having a mass approximately 1/1836 that of a proton. They are considered point particles, meaning they have no measurable size. This property makes electrons challenging to study and understand fully within the framework of current physical theories.
Electron Shells and Energy Levels
Electrons don't orbit the nucleus in a simple, predictable manner as depicted in older models. Instead, they occupy regions of space around the nucleus called electron shells or energy levels. These shells represent different energy states that electrons can occupy. Electrons in lower energy levels are closer to the nucleus, while electrons in higher energy levels are further away. The arrangement of electrons in these shells determines the chemical properties of an atom and its ability to form chemical bonds with other atoms.
Electron Interactions and Chemical Bonding
The electrons of an atom are primarily responsible for its chemical behavior. Electrons in the outermost shell, known as valence electrons, participate in chemical bonding. Atoms interact with each other by sharing or transferring valence electrons to form molecules and compounds. This transfer or sharing of electrons leads to the formation of chemical bonds, which are responsible for the diversity of matter and the complexity of chemical reactions.
The Interplay of Subatomic Particles and Atomic Structure
The three subatomic particles – protons, neutrons, and electrons – interact to form atoms, the fundamental building blocks of matter. The number of protons defines the element, while the number of neutrons determines the isotope. The electrons, in their various energy levels, dictate the atom's chemical properties and its ability to interact with other atoms. The electromagnetic force governs the interactions between charged particles (protons and electrons), while the strong nuclear force holds the nucleus together.
Beyond the Basics: The Expanding World of Subatomic Particles
While protons, neutrons, and electrons provide a foundational understanding of atomic structure, the world of subatomic particles is much more complex. Protons and neutrons are themselves composed of even smaller particles called quarks, held together by the strong force mediated by gluons. Beyond these particles, there exists a vast zoo of other particles discovered through particle accelerators, each with its own unique properties and interactions. This ongoing research into subatomic particles continues to deepen our understanding of the fundamental forces and building blocks of the universe. However, protons, neutrons and electrons remain the critical components of ordinary matter that surrounds us daily.
Implications for Various Fields
Understanding the nature and behavior of these subatomic particles is fundamental to many scientific fields:
- Chemistry: The arrangement and interactions of electrons dictate chemical bonding, reactivity, and the properties of molecules.
- Nuclear Physics: The study of protons and neutrons is crucial to understanding nuclear reactions, nuclear energy, and radioactive decay.
- Materials Science: The properties of materials are ultimately determined by the interactions of their constituent atoms, governed by the behavior of subatomic particles.
- Electronics: The behavior of electrons in conductors and semiconductors is essential to the functioning of electronic devices.
- Cosmology and Astrophysics: The properties and interactions of subatomic particles play a crucial role in understanding the formation and evolution of stars, galaxies, and the universe as a whole.
In conclusion, the three subatomic particles – protons (positive charge), neutrons (neutral charge), and electrons (negative charge) – are fundamental to our understanding of matter. Their properties and interactions define the structure and behavior of atoms, molecules, and all the materials around us. While the exploration of subatomic particles continues, these three remain the key to understanding the world at its most basic level, impacting numerous scientific disciplines and technological advancements.
Latest Posts
Latest Posts
-
What Is The Most Reactive Group On The Periodic Table
Apr 02, 2025
-
What Is 3 Divided By 11
Apr 02, 2025
-
How To Find The Mass Of The Excess Reactant
Apr 02, 2025
-
Instantaneous Rate Of Change Vs Average Rate Of Change
Apr 02, 2025
-
How Many D Orbitals Can Be In An Energy Level
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Are 3 Subatomic Particles And Their Charges . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
