What Are 3 Parts Of An Atp Molecule
listenit
Mar 23, 2025 · 5 min read
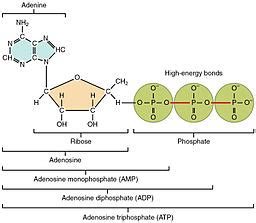
Table of Contents
What Are the 3 Parts of an ATP Molecule? Unlocking the Energy Currency of Life
Adenosine triphosphate (ATP) is the fundamental energy currency of all living cells. This molecule fuels countless cellular processes, from muscle contraction and nerve impulse transmission to protein synthesis and DNA replication. Understanding its structure is key to understanding how life itself functions. This article delves deep into the three essential components of an ATP molecule, exploring their individual roles and their synergistic contribution to ATP's remarkable energy-storing capabilities.
The Tripartite Structure of ATP: Adenine, Ribose, and Triphosphate
The ATP molecule is a relatively small but incredibly complex structure, composed of three distinct parts:
- Adenine: A nitrogenous base belonging to the purine family.
- Ribose: A five-carbon sugar (pentose).
- Triphosphate: A chain of three phosphate groups linked together.
Let's examine each component in detail:
1. Adenine: The Nitrogenous Base
Adenine is a crucial component not only of ATP but also of other vital molecules like DNA and RNA. Its structure consists of a six-membered ring fused to a five-membered ring, both containing nitrogen atoms. These nitrogen atoms are crucial for adenine's ability to form hydrogen bonds, particularly with thymine (in DNA) or uracil (in RNA), which are essential for the structural integrity and informational storage capabilities of these nucleic acids. In ATP, adenine's role is primarily structural, contributing to the overall shape and stability of the molecule. It doesn't directly participate in the energy transfer process but is a vital part of the whole. Think of it as the anchor point for the energy-rich phosphate groups.
The Role of Adenine in ATP's Functionality: Adenine provides the specific molecular recognition site. This is important for the enzymes that interact with ATP, ensuring that ATP is utilized by the correct enzymes for specific cellular processes. Without the unique structural features of adenine, ATP wouldn't be able to bind correctly to the enzymes responsible for ATP hydrolysis and phosphorylation.
2. Ribose: The Sugar Backbone
Ribose, a five-carbon sugar, forms the backbone of the ATP molecule. Specifically, it's a β-D-ribose, meaning it's a ribose molecule in its beta-anomeric form and it is a D-sugar (a member of the D-family of sugars). The arrangement of its carbon atoms and hydroxyl groups is crucial for its interaction with the adenine base and the triphosphate group. The ribose molecule is a crucial structural component, connecting adenine and the phosphate chain, providing the essential framework for the molecule's overall configuration and stability. The hydroxyl groups on ribose also play a role in the chemical reactions involving ATP.
Ribose's Impact on ATP Stability and Reactivity: The specific structure of ribose dictates the molecule's conformation, influencing the accessibility and reactivity of the phosphate groups. The placement of the hydroxyl groups allows for specific interactions with water molecules and other cellular components, thus impacting its solubility and stability within the cellular environment.
3. Triphosphate: The Energy-Rich Tail
The triphosphate group is undoubtedly the most critical aspect of the ATP molecule. This chain of three phosphate groups (α, β, and γ) is linked together via high-energy phosphoanhydride bonds. These are not typical covalent bonds; they are considerably less stable than the phosphoester bonds found in other molecules due to repulsion between the negatively charged oxygen atoms of adjacent phosphate groups. This inherent instability is what makes the triphosphate group the driving force behind ATP's energy-storage capacity. The energy released during the hydrolysis of these bonds powers numerous cellular processes.
The High-Energy Phosphoanhydride Bonds: The energy is not stored within the bonds themselves in the form of potential energy like a stretched spring, but rather the energy is released due to the increased stability and lower energy state of the products of the hydrolysis reaction compared to the reactants. This is a result of resonance stabilization in the products, increased entropy, and favorable hydration of the products.
Hydrolysis and Energy Release: The breaking of these phosphoanhydride bonds, typically between the β and γ phosphates (yielding ADP and inorganic phosphate, Pi), releases a significant amount of free energy. This energy is harnessed by enzymes to drive various endergonic (energy-requiring) reactions within the cell. The process is often coupled—the exergonic (energy-releasing) hydrolysis of ATP provides the energy needed to drive the endergonic process.
ATP Regeneration: It is crucial to remember that ATP is not a static energy store; it's constantly being synthesized and hydrolyzed. The majority of ATP is regenerated through cellular respiration, a metabolic pathway that breaks down glucose and other fuel molecules to generate ATP. Other processes like substrate-level phosphorylation also contribute to ATP regeneration.
ATP's Role as the Cellular Energy Currency
The unique structure of ATP, with its three distinct parts working in concert, makes it an exceptionally efficient energy carrier. The high-energy bonds in the triphosphate group readily release energy upon hydrolysis, fueling countless cellular processes. This energy is used for:
- Muscle Contraction: ATP powers the myosin-actin interaction in muscle cells, leading to muscle movement.
- Nerve Impulse Transmission: The transmission of nerve impulses relies on the movement of ions across cell membranes, a process requiring ATP.
- Active Transport: ATP drives active transport processes, moving molecules against their concentration gradient across cell membranes.
- Protein Synthesis: The synthesis of proteins requires energy to form peptide bonds between amino acids, a process that utilizes ATP.
- DNA Replication and Repair: These fundamental processes require energy to unwind DNA strands and synthesize new DNA molecules, relying heavily on ATP.
- Biosynthesis: The synthesis of various molecules, including lipids, carbohydrates, and nucleotides, require ATP to drive the endergonic reactions involved.
Conclusion: A Masterpiece of Molecular Design
The three parts of an ATP molecule—adenine, ribose, and the triphosphate group—are intricately interconnected, forming a remarkably efficient energy carrier. Adenine and ribose provide the structural backbone, while the triphosphate group stores and readily releases energy through hydrolysis of its high-energy phosphoanhydride bonds. This elegant design allows ATP to serve as the fundamental energy currency of life, powering the countless processes that keep cells alive and functioning. Understanding this tripartite structure is essential for comprehending the intricate workings of cellular metabolism and the remarkable efficiency of life itself. Further research into the precise interactions between ATP and the enzymes that utilize it continues to reveal more about the intricacies of cellular energy transfer and regulation. The seemingly simple structure of ATP belies its fundamental role in the complex symphony of life.
Latest Posts
Latest Posts
-
How Many Electrons In 3rd Energy Level
Mar 25, 2025
-
All Real Square Roots Of 4
Mar 25, 2025
-
Common Factors Of 36 And 24
Mar 25, 2025
-
What Is The Electron Configuration For Gallium
Mar 25, 2025
-
What Percentage Of 36 Is 24
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Are 3 Parts Of An Atp Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
