What Is The Electron Configuration For Gallium
listenit
Mar 25, 2025 · 5 min read
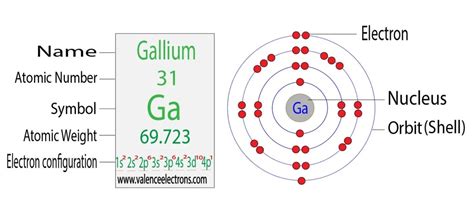
Table of Contents
What is the Electron Configuration for Gallium? A Deep Dive into Atomic Structure
Gallium, a fascinating element with a unique set of properties, finds widespread applications in various fields, from semiconductors to medicine. Understanding its atomic structure, particularly its electron configuration, is crucial to comprehending its behavior and applications. This article will delve deep into the electron configuration of gallium, exploring its implications and relating it to the element's characteristics.
Understanding Electron Configuration
Before we dive into gallium's specific configuration, let's establish a basic understanding of what electron configuration represents. An electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. This arrangement dictates an atom's chemical properties, its reactivity, and its bonding behavior. It follows the Aufbau principle, which states that electrons fill orbitals in order of increasing energy, and the Pauli exclusion principle, which dictates that each orbital can hold a maximum of two electrons with opposite spins. Hund's rule further specifies that electrons will individually occupy orbitals within a subshell before pairing up.
The electron configuration is usually represented using a notation that specifies the principal quantum number (n), the subshell (s, p, d, or f), and the number of electrons in each subshell. For example, 1s² indicates two electrons in the 1s subshell.
Determining the Electron Configuration of Gallium (Ga)
Gallium (Ga) has an atomic number of 31, meaning it possesses 31 protons and, in its neutral state, 31 electrons. To determine its electron configuration, we follow the Aufbau principle, filling the orbitals in increasing energy order. The order of filling is generally:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p...
Applying this to gallium's 31 electrons, we obtain the following electron configuration:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p¹
This configuration can also be represented in a shorthand notation using the noble gas configuration. The noble gas preceding gallium is Argon (Ar), which has the electron configuration 1s² 2s² 2p⁶ 3s² 3p⁶. Therefore, the shorthand notation for gallium's electron configuration is:
[Ar] 3d¹⁰ 4s² 4p¹
This shorthand notation is often preferred for its brevity and clarity. It highlights the core electrons (those shared with Argon) and focuses on the valence electrons, which are responsible for the element's chemical reactivity.
The Significance of Gallium's Valence Electrons
The outermost electrons, the valence electrons, are crucial in determining an element's chemical behavior. In gallium's case, the valence electrons are located in the 4s and 4p orbitals. Specifically, it has three valence electrons (4s² 4p¹). This configuration explains why gallium is a group 13 element (also known as group IIIA) and exhibits a +3 oxidation state in many of its compounds. These three valence electrons are readily available to participate in chemical bonding.
Relating Electron Configuration to Gallium's Properties
Gallium's electron configuration is intrinsically linked to its physical and chemical properties. Let's examine some key aspects:
1. Metallic Character:
The presence of valence electrons indicates Gallium's metallic character. These loosely held electrons are readily delocalized, contributing to gallium's excellent electrical and thermal conductivity. This is a typical characteristic of metals.
2. Melting Point and Boiling Point:
Gallium's relatively low melting point (29.76 °C) compared to other metals is partially attributed to the weak metallic bonding associated with its electron configuration. The weak interaction between its atoms results in a relatively low energy requirement to transition from the solid to liquid state. However, it has a surprisingly high boiling point.
3. Chemical Reactivity:
The three valence electrons explain gallium's reactivity. It readily forms compounds by losing these three electrons to achieve a stable, noble gas configuration. This explains its common +3 oxidation state. It can also display a +1 oxidation state in some compounds, a phenomenon less common in other group 13 elements and often attributed to the relativistic effects on the 4s and 4p electrons.
4. Semiconductor Properties:
Gallium's electron configuration and its ability to form compounds with elements like arsenic (GaAs) are crucial to its applications in semiconductors. The precise arrangement of electrons allows for controlled conductivity, making it a valuable component in electronic devices. The electron configuration contributes to the band gap characteristics essential for semiconductor applications.
5. Applications:
Gallium's unique properties, directly related to its electron configuration, have led to its widespread use in diverse applications:
- Semiconductors: Gallium arsenide (GaAs) and other gallium compounds are essential in high-speed transistors, solar cells, and light-emitting diodes (LEDs).
- Medicine: Gallium-67 is a radioisotope used in medical imaging.
- Alloys: Gallium is used in low-melting alloys, finding applications in temperature sensors and other specialized materials.
- Mirrors: Its high reflectivity makes it suitable for high-quality mirrors.
Exceptions and Anomalies
While the Aufbau principle provides a general guideline for electron configuration, exceptions can occur. The filling of orbitals is influenced by various factors, including electron-electron repulsion and relativistic effects, particularly for heavier elements. While gallium’s configuration follows the general rule, it’s crucial to remember that the electron configurations of elements can sometimes deviate slightly from the predicted order.
Advanced Concepts and Further Exploration
For a more advanced understanding, exploring concepts like:
- Relativistic effects: These effects become more significant for heavier elements like gallium and influence the energy levels of electrons.
- Slater's rules: These rules provide a more refined approach to calculating effective nuclear charge and understanding electron shielding.
- Quantum mechanical calculations: Advanced computational methods can provide highly accurate electron configurations and energy levels.
Conclusion
The electron configuration of gallium, 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p¹ or [Ar] 3d¹⁰ 4s² 4p¹, is fundamental to understanding its unique properties and wide-ranging applications. The three valence electrons are key to its reactivity, metallic character, and ability to form crucial compounds used in semiconductors and other technologies. While the Aufbau principle provides a solid foundation, deeper exploration into relativistic effects and advanced quantum mechanics is necessary for a complete picture of its atomic structure. Understanding gallium's electron configuration provides a crucial stepping stone for further study in chemistry, materials science, and related fields. The information presented here serves as a comprehensive overview, inviting further investigation and a deeper appreciation for this fascinating element's intricate atomic structure.
Latest Posts
Latest Posts
-
What Is The Least Common Multiple Of 9 And 5
Mar 26, 2025
-
Derivative Of Square Root Of Xy
Mar 26, 2025
-
What Is 10 5 As A Decimal
Mar 26, 2025
-
What Are The Factors For 43
Mar 26, 2025
-
How To Convert Polar Equations To Rectangular Form
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Gallium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
