How Many Electrons In 3rd Energy Level
listenit
Mar 25, 2025 · 5 min read
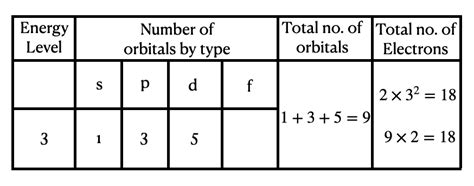
Table of Contents
- How Many Electrons In 3rd Energy Level
- Table of Contents
- How Many Electrons in the 3rd Energy Level? Unveiling the Secrets of Electron Shells
- The Quantum Mechanical Model and Electron Shells
- Subshells and Orbital Occupancy
- Calculating the Electron Capacity of the 3rd Energy Level
- Electron Configuration and the Aufbau Principle
- Implications for Chemical Properties
- Exceptions to the Rule and Orbital Interactions
- Conclusion: The Significance of the 3rd Energy Level
- Latest Posts
- Latest Posts
- Related Post
How Many Electrons in the 3rd Energy Level? Unveiling the Secrets of Electron Shells
Understanding electron configuration is fundamental to comprehending the behavior of atoms and the properties of matter. A key aspect of this understanding involves knowing how many electrons can occupy each energy level, or shell, within an atom. This article delves deep into the question: how many electrons are in the 3rd energy level? We'll explore the underlying principles of electron arrangement, the significance of quantum numbers, and the implications of electron shell filling for chemical reactivity and atomic properties.
The Quantum Mechanical Model and Electron Shells
Unlike the simplified Bohr model, the more accurate quantum mechanical model describes electrons not as orbiting the nucleus in neat circles, but as existing in regions of space called orbitals. These orbitals are defined by a set of quantum numbers, each carrying specific information about an electron's state. These numbers are:
-
Principal Quantum Number (n): This number designates the electron shell or energy level.
ncan be any positive integer (1, 2, 3, ...). Higher values ofnindicate higher energy levels and greater distances from the nucleus. The 3rd energy level, therefore, hasn = 3. -
Azimuthal Quantum Number (l): This number specifies the subshell within a given energy level. It can range from 0 to
n-1. For the 3rd energy level (n = 3),lcan be 0, 1, or 2, corresponding to the s, p, and d subshells, respectively. -
Magnetic Quantum Number (ml): This number describes the orientation of the orbital in space. It ranges from
-lto+l, including 0. -
Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron, with values of +1/2 or -1/2, often represented as "spin up" and "spin down."
Subshells and Orbital Occupancy
The number of electrons in an energy level isn't simply determined by the principal quantum number alone. The different subshells within a level contribute to the total electron count. Each subshell can hold a specific number of electrons:
- s subshell (l=0): One orbital, holding a maximum of 2 electrons (one spin up and one spin down).
- p subshell (l=1): Three orbitals, holding a maximum of 6 electrons (2 electrons per orbital).
- d subshell (l=2): Five orbitals, holding a maximum of 10 electrons (2 electrons per orbital).
- f subshell (l=3): Seven orbitals, holding a maximum of 14 electrons (2 electrons per orbital).
Calculating the Electron Capacity of the 3rd Energy Level
Now, let's apply this to the 3rd energy level (n=3). Since n=3, the possible values for l are 0, 1, and 2, representing the 3s, 3p, and 3d subshells.
- 3s subshell: Holds a maximum of 2 electrons.
- 3p subshell: Holds a maximum of 6 electrons.
- 3d subshell: Holds a maximum of 10 electrons.
Therefore, the total maximum number of electrons that can occupy the 3rd energy level is the sum of the electrons in each subshell: 2 + 6 + 10 = 18 electrons.
Electron Configuration and the Aufbau Principle
The way electrons fill the energy levels and subshells follows the Aufbau principle, which states that electrons first fill the lowest energy levels available. The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on. However, there are exceptions to this rule due to subtle energy differences between subshells.
For example, consider the element Argon (Ar), with atomic number 18. Its electron configuration is 1s²2s²2p⁶3s²3p⁶. Notice that all 18 electrons are accommodated within the first three energy levels, with the 3rd energy level containing 8 electrons (2 in 3s and 6 in 3p). This is because the 4s subshell has a slightly lower energy than the 3d subshell, leading to its filling before the 3d subshell.
Elements beyond Argon will begin filling the 3d subshell, eventually leading to a completely filled 3rd energy level with 18 electrons in elements such as Zinc (Zn).
Implications for Chemical Properties
The number of electrons in the outermost energy level, known as the valence electrons, plays a crucial role in determining an atom's chemical reactivity. For elements in the third period (row) of the periodic table, the valence electrons are those in the 3s and 3p subshells. The varying numbers of valence electrons influence how these atoms form chemical bonds and participate in chemical reactions.
Exceptions to the Rule and Orbital Interactions
While the Aufbau principle provides a good general guideline, exceptions exist. Electron-electron repulsions and the subtle energy differences between subshells can cause slight deviations in the filling order. These deviations are often observed in transition metals and lanthanides, where the energy levels are closely spaced. The precise electron configuration is sometimes best determined experimentally using spectroscopic techniques.
Conclusion: The Significance of the 3rd Energy Level
The 3rd energy level, capable of holding up to 18 electrons, is a vital component of atomic structure. Understanding its electron capacity and how electrons fill its subshells is key to predicting the chemical and physical properties of elements. The arrangement of electrons in this and other energy levels dictates how atoms interact, forming molecules, compounds, and the diverse materials that make up our world. While the simple answer is 18 electrons, the intricacies of quantum mechanics and the nuances of electron configurations offer a much richer and more fascinating understanding of the atomic world. By mastering these concepts, we gain a deeper appreciation of the fundamental building blocks of matter and the forces that govern their behavior. Further exploration into advanced quantum chemistry can reveal even greater detail about electron behavior within the 3rd and other energy levels.
Latest Posts
Latest Posts
-
Does Side Side Angle Prove Congruence
Mar 27, 2025
-
Is Pi 2 A Rational Number
Mar 27, 2025
-
How Much Is 1 4 In Ounces
Mar 27, 2025
-
X 4 3x 2 4 0
Mar 27, 2025
-
How Many Lines Of Symmetry In A Rectangle
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons In 3rd Energy Level . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
