Weight Of 1 Cubic Meter Water
listenit
Mar 26, 2025 · 5 min read
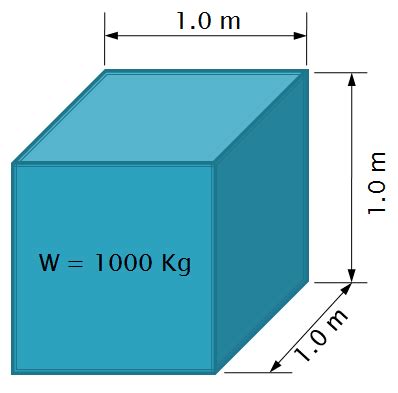
Table of Contents
The Weight of 1 Cubic Meter of Water: A Deep Dive
The seemingly simple question, "What is the weight of 1 cubic meter of water?" opens a door to a fascinating exploration of density, temperature, purity, and the very nature of water itself. While a quick Google search might give you a straightforward answer, the reality is far more nuanced and intriguing. This article will delve into the complexities surrounding the weight of a cubic meter of water, exploring the factors that influence it and its practical applications across various fields.
Understanding Density and its Role
Before we jump into the weight calculation, it's crucial to understand the concept of density. Density is defined as the mass of a substance per unit volume. For water, this is typically expressed as kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³). The relationship between mass, volume, and density is given by the simple formula:
Density = Mass / Volume
This formula is fundamental to determining the weight of 1 cubic meter of water. However, the weight itself isn't solely determined by the density. It's also influenced by the force of gravity acting on that mass. Weight, therefore, is the force exerted on an object due to gravity. The formula for weight is:
Weight = Mass × Gravity
Where gravity is approximately 9.81 m/s² on the Earth's surface.
The Standard Weight: 1000 kg
Under standard conditions – specifically, at a temperature of 4° Celsius (39.2° Fahrenheit) and at standard atmospheric pressure – the density of pure water is approximately 1000 kg/m³. This means that 1 cubic meter of pure water at this temperature weighs approximately 1000 kg, or 1 metric ton. This is often used as a benchmark figure, but it's crucial to remember this is an idealized value.
Why 4°C?
The reason 4°C is chosen as the standard temperature is because this is the temperature at which water reaches its maximum density. Above and below this temperature, the density of water slightly decreases. This anomalous behavior of water is critical to aquatic life, preventing bodies of water from freezing solid from the bottom up.
Factors Affecting the Weight
Several factors can significantly influence the weight of 1 cubic meter of water, causing deviations from the standard 1000 kg figure. Let's explore some key influencers:
1. Temperature: The Thermal Effect
As mentioned, temperature plays a crucial role. Water expands as it heats up and contracts as it cools. This change in volume directly affects the density, and consequently, the weight. Water at 0°C (32°F) is slightly less dense, and therefore weighs slightly less than water at 4°C. Similarly, warmer water is less dense and weighs less. This thermal effect needs careful consideration in many applications, such as hydrological studies and industrial processes.
2. Salinity: The Salty Difference
The presence of dissolved salts significantly alters the density of water. Seawater, for example, is denser than freshwater due to the dissolved salts. The higher the salinity, the higher the density and consequently, the higher the weight of a cubic meter of water. Oceanographers and marine scientists must account for salinity variations when making accurate weight calculations related to ocean currents and marine ecosystems.
3. Pressure: Deep Sea Density
Pressure also affects the density of water. At greater depths, the pressure increases, causing water molecules to pack more tightly together. This leads to a slightly higher density and therefore a slightly higher weight for a given volume. While the effect isn't dramatic at relatively shallow depths, it becomes increasingly significant in the deep ocean. This is a crucial factor in oceanographic modeling and understanding deep-sea ecosystems.
4. Impurities: Beyond Pure Water
The presence of impurities, such as dissolved minerals, sediments, or pollutants, affects the water's density and weight. These impurities can increase or decrease the density depending on their nature and concentration. For instance, the presence of heavy metals could increase the density, while certain organic matter might reduce it. Accurate measurement of water purity is essential in many industries like water treatment and pharmaceuticals.
5. Altitude: The Gravitational Pull
While less significant than the previous factors, the gravitational pull varies slightly with altitude. At higher altitudes, the gravitational acceleration is slightly lower, resulting in a slightly lower weight for a given mass of water. This effect is typically negligible in most practical applications but becomes relevant in high-precision measurements or studies involving significant altitude changes.
Practical Applications: Weighing the Significance
The weight of 1 cubic meter of water has profound implications across various fields:
1. Hydrology and Meteorology: Water Resource Management
Understanding the weight and density of water is crucial for hydrological modeling, forecasting floods, managing water resources, and studying the water cycle. Accurate weight calculations are essential for designing dams, irrigation systems, and other water management infrastructure.
2. Oceanography: Exploring the Depths
In oceanography, the density and weight of water are critical for studying ocean currents, stratification, and mixing processes. The variations in density due to temperature, salinity, and pressure drive these crucial oceanographic phenomena. This knowledge contributes to our understanding of marine ecosystems and climate change impacts.
3. Civil Engineering: Structural Design
Civil engineers need to consider the weight of water when designing structures such as dams, bridges, and pipelines. Accurate weight calculations are essential for ensuring the structural integrity and safety of these large-scale projects.
4. Chemical Engineering: Process Optimization
Chemical engineers utilize the density and weight of water in various process designs, particularly those involving fluid dynamics and heat transfer. Accurate estimations are crucial for optimizing chemical reactions, separation processes, and transportation systems.
5. Environmental Science: Pollution Monitoring
The density and weight of water play a vital role in environmental monitoring and pollution assessment. Water quality parameters often rely on measurements that depend on density and weight, enabling accurate pollution assessments and effective environmental management.
Conclusion: Beyond the Simple Answer
While the simple answer to the weight of 1 cubic meter of water is often given as 1000 kg, the reality is much more complex. This article highlights the various factors that can influence this weight, including temperature, salinity, pressure, impurities, and altitude. Understanding these influences is crucial for accurate calculations and practical applications across numerous scientific and engineering disciplines. Accurate measurements and a thorough understanding of the interplay between these factors are vital for making informed decisions in water resource management, oceanographic research, civil engineering, chemical processing, and environmental science. The seemingly simple question, therefore, unlocks a wealth of knowledge and practical significance.
Latest Posts
Latest Posts
-
How To Find Number Of Core Electrons
Mar 28, 2025
-
What Is The Formula For The Compound Magnesium Oxide
Mar 28, 2025
-
What Is The Correct Formula For Calcium Oxide
Mar 28, 2025
-
What Is The Si Base Unit Of Length
Mar 28, 2025
-
What Is The Oxidation State Of Each Element In Coh2
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Weight Of 1 Cubic Meter Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
