The Number Of Protons In The Nucleus Of An Element
listenit
Mar 22, 2025 · 6 min read
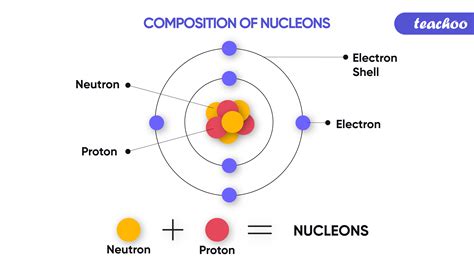
Table of Contents
The Number of Protons: The Defining Feature of an Element
The number of protons in the nucleus of an atom is arguably the single most important characteristic defining a chemical element. This fundamental property dictates the element's position on the periodic table, its chemical behavior, and its interaction with other elements. Understanding the number of protons, also known as the atomic number, is crucial to comprehending the very building blocks of matter. This article delves deep into the significance of this number, exploring its implications in various fields of science and providing a comprehensive overview of its role in shaping our understanding of the universe.
The Atomic Number: The Identity Card of an Element
Every atom, the basic unit of matter, consists of a nucleus surrounded by electrons. The nucleus itself contains two types of particles: protons and neutrons. Protons carry a positive electrical charge, while neutrons are electrically neutral. The atomic number (Z) of an element is simply the number of protons found in the nucleus of a single atom of that element. This number is unique to each element and doesn't change under normal chemical conditions.
Why is the Atomic Number so Important?
The atomic number's significance stems from its direct correlation to several key characteristics:
-
Element Identity: The atomic number is the primary identifier of an element. Each element possesses a unique atomic number; no two elements share the same number of protons. For instance, hydrogen (H) has an atomic number of 1 (one proton), helium (He) has an atomic number of 2 (two protons), and so on. This is the fundamental principle that organizes the periodic table.
-
Chemical Properties: The number of protons determines the number of electrons an atom possesses in its neutral state. These electrons are responsible for chemical bonding, defining how an element interacts with other elements to form molecules and compounds. Elements with similar numbers of electrons in their outermost shell (valence electrons) exhibit similar chemical behaviors, a pattern reflected in the periodic table's arrangement of groups and periods.
-
Isotopes: While the number of protons defines the element, the number of neutrons can vary. Atoms of the same element with differing numbers of neutrons are called isotopes. Isotopes of an element have the same atomic number but different mass numbers (the sum of protons and neutrons). For example, carbon-12 and carbon-14 are isotopes of carbon, both having 6 protons but differing in their neutron count (6 and 8, respectively). Some isotopes are stable, while others are radioactive, undergoing decay to transform into different elements.
-
Periodic Table Organization: The periodic table is arranged in increasing order of atomic number. This systematic arrangement beautifully showcases the periodic trends in elemental properties. Elements with similar electronic configurations (and thus similar chemical properties) are placed in the same group (column), while elements within the same period (row) have the same number of electron shells.
Determining the Atomic Number: Methods and Techniques
Several techniques are used to determine the atomic number of an element, ranging from simple observations to sophisticated spectroscopic methods:
-
X-ray Spectroscopy: This technique exploits the fact that the energy of X-rays emitted by an element is characteristic of its atomic number. By analyzing the emitted X-rays, scientists can precisely determine the number of protons in the atom.
-
Mass Spectrometry: This powerful technique separates ions based on their mass-to-charge ratio. While it doesn't directly measure the atomic number, it can determine the isotopic composition of an element, allowing the calculation of the atomic number from the mass number and neutron count.
-
Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy provides information about the atomic environment of an element, which can indirectly aid in determining the atomic number. Although not a primary method, the chemical shifts and coupling constants obtained can assist in identifying the element and consequently its atomic number.
-
Chemical Reactions and Analysis: Qualitative and quantitative chemical analyses based on the reactivity and behavior of an element can be used to infer the atomic number. This method relies on extensive knowledge of chemical properties and periodic trends.
The Atomic Number and its Applications
The knowledge of atomic numbers has far-reaching implications across various scientific disciplines:
Chemistry:
-
Predicting Chemical Reactions: Understanding atomic numbers helps predict how elements will react with each other, forming the basis for synthetic chemistry, the design and synthesis of new molecules with specific properties.
-
Molecular Structure Determination: The atomic number directly influences the electronic structure of an atom, enabling scientists to determine molecular structures and predict their properties based on electron configuration and bonding patterns.
-
Catalysis: The use of specific elements as catalysts relies heavily on understanding their atomic numbers and associated electronic properties, making it possible to enhance the rates of chemical reactions.
Nuclear Physics:
-
Nuclear Reactions: In nuclear reactions, changes in the number of protons lead to the formation of new elements through processes like fission and fusion. Understanding the atomic number is fundamental to modeling and controlling these reactions.
-
Radioactive Decay: Radioactive isotopes, differentiated by their neutron counts while maintaining the same atomic number, undergo decay processes which are critical in various applications like medical imaging, dating techniques, and industrial gauging.
-
Nuclear Medicine: Radioactive isotopes with specific atomic numbers are used in nuclear medicine for diagnostic imaging (e.g., PET scans) and cancer therapy.
Material Science:
-
Material Properties: The atomic number significantly influences the physical and chemical properties of materials. Knowing the atomic number of constituent elements enables predicting and tailoring material properties for desired applications.
-
Alloy Design: The creation of alloys involves combining elements with specific atomic numbers to achieve desired mechanical, electrical, or magnetic properties.
-
Semiconductor Technology: The properties of semiconductors, crucial for electronics, depend on the precise number of protons and electrons in the constituent elements.
Astrophysics:
-
Stellar Nucleosynthesis: The creation of elements within stars through nuclear fusion processes is determined by the atomic numbers of the involved elements.
-
Spectroscopy of Stars and Galaxies: The light emitted by stars contains spectral lines characteristic of the elements present, enabling astronomers to determine the elemental composition of celestial objects based on atomic numbers.
Beyond the Atomic Number: Isotopes and Nuclear Stability
While the atomic number uniquely identifies an element, the number of neutrons in the nucleus (along with the number of protons, contributing to the mass number) profoundly influences the atom's stability. Isotopes, atoms of the same element with varying neutron counts, exhibit different stabilities. Some isotopes are stable, while others are radioactive, undergoing decay to achieve a more stable configuration. The stability of an isotope is determined by the balance between the strong nuclear force (holding the nucleus together) and the electromagnetic force (causing protons to repel each other). This balance affects the half-life of radioactive isotopes, which ranges from fractions of a second to billions of years.
Conclusion: The Unshakeable Foundation of Chemistry and Beyond
The number of protons in an atom's nucleus, the atomic number, serves as the cornerstone of our understanding of the chemical elements and their behavior. It is the defining characteristic of an element, dictating its position in the periodic table, its chemical properties, and its interactions with other elements. The profound implications of atomic number extend far beyond the realm of chemistry, impacting diverse fields such as nuclear physics, material science, and astrophysics. Its importance is undeniable, and its continued study remains essential for advancing our knowledge of the universe and harnessing the power of matter. Understanding atomic numbers is not just about memorizing numbers; it's about understanding the fundamental building blocks of reality. This knowledge empowers us to predict, manipulate, and utilize the properties of matter for countless scientific and technological advancements.
Latest Posts
Latest Posts
-
What Are 3 Parts Of An Atp Molecule
Mar 23, 2025
-
Draw The Electron Configuration For A Neutral Atom Of Iron
Mar 23, 2025
-
Each Column In The Periodic Table Is Called A
Mar 23, 2025
-
How Many Ounces In 3 4
Mar 23, 2025
-
4 To The Power Of Negative 2
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about The Number Of Protons In The Nucleus Of An Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
