Draw The Electron Configuration For A Neutral Atom Of Iron.
listenit
Mar 23, 2025 · 5 min read
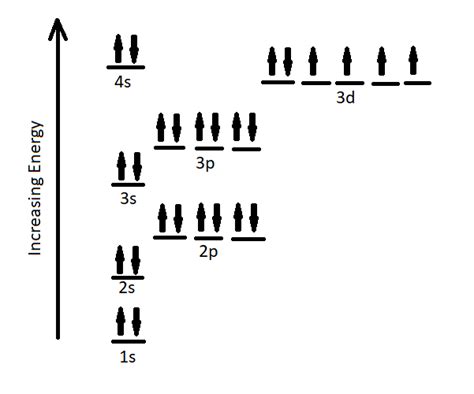
Table of Contents
Drawing the Electron Configuration for a Neutral Atom of Iron: A Comprehensive Guide
Iron, a ubiquitous element vital to life and industry, presents a fascinating case study in electron configuration. Understanding its electron arrangement unlocks insights into its chemical properties and behaviour. This comprehensive guide will delve into the process of drawing the electron configuration for a neutral iron atom, explaining the underlying principles and offering a detailed step-by-step approach. We'll also explore related concepts like orbital diagrams and Hund's rule to provide a complete picture of iron's electronic structure.
Understanding Electron Configuration
Electron configuration describes the arrangement of electrons in an atom's energy levels and sublevels. It's a fundamental concept in chemistry, crucial for predicting an element's reactivity and other properties. Electrons occupy orbitals, regions within an atom where the probability of finding an electron is high. These orbitals are organized into shells (represented by the principal quantum number, n), subshells (represented by the azimuthal quantum number, l), and individual orbitals within subshells.
The Aufbau Principle and Hund's Rule
Two key principles govern electron configuration:
-
The Aufbau Principle: Electrons fill orbitals starting from the lowest energy level and progressing upwards. This is like building a house – you start with the foundation before adding the upper floors.
-
Hund's Rule: Within a subshell, electrons initially occupy orbitals individually before pairing up. This minimizes electron-electron repulsion, leading to a more stable configuration. Think of it as students preferring single rooms in a dorm before sharing.
Determining the Electron Configuration of Iron (Fe)
Iron (Fe) has an atomic number of 26, meaning a neutral iron atom possesses 26 electrons. To determine its electron configuration, we'll follow the Aufbau principle and Hund's rule, using the standard notation:
-
Identify the number of electrons: Iron has 26 electrons.
-
Fill the subshells in order of increasing energy: The order is typically represented using the Aufbau principle diagram, but we can also use the following sequence: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... and so on.
-
Determine the number of electrons in each subshell: Each subshell can hold a specific number of electrons: s subshell holds 2 electrons, p subshell holds 6 electrons, d subshell holds 10 electrons, and f subshell holds 14 electrons.
-
Apply the Aufbau principle and Hund's rule: We fill the subshells according to the energy level and using Hund's rule for electron pairing.
Let's apply this to iron:
- 1s²: The first shell (n=1) has one subshell (s), which holds 2 electrons.
- 2s²: The second shell (n=2) starts with the s subshell, holding 2 electrons.
- 2p⁶: The second shell also has a p subshell, which can hold 6 electrons.
- 3s²: The third shell (n=3) begins with the s subshell, holding 2 electrons.
- 3p⁶: The third shell's p subshell can hold 6 electrons.
- 4s²: The fourth shell (n=4) starts with the s subshell, holding 2 electrons.
- 3d⁶: Finally, we reach the 3d subshell in the third shell. It can hold up to 10 electrons, but iron only has 6 electrons remaining.
Therefore, the complete electron configuration of a neutral iron atom is: 1s²2s²2p⁶3s²3p⁶4s²3d⁶.
Orbital Diagrams and Hund's Rule Visualization
While the electron configuration notation is concise, orbital diagrams provide a more visual representation, especially when illustrating Hund's rule. Each orbital is represented by a box, and electrons are represented by arrows. Up and down arrows signify electrons with opposite spins.
For iron's 3d subshell, Hund's rule dictates that the six electrons occupy individual orbitals before pairing up. This results in four orbitals with one electron each and one orbital with two electrons (a pair). This is crucial for understanding iron's magnetic properties.
The orbital diagram for iron's 3d subshell would show five boxes (representing the five 3d orbitals), with four boxes containing one upward-pointing arrow each and one box containing an upward and a downward-pointing arrow. This visualization clearly demonstrates the application of Hund's rule.
Exceptions to the Aufbau Principle
While the Aufbau principle provides a general framework, some elements deviate from the predicted electron configuration. This is primarily due to the subtle energy differences between subshells, especially those close in energy. Iron itself doesn't exhibit a significant exception, but understanding this possibility is important for a complete understanding. These exceptions often involve the filling of d and f subshells.
Iron's Electron Configuration and its Properties
The specific electron configuration of iron is directly responsible for its properties. The presence of unpaired electrons in the 3d subshell explains iron's ferromagnetism, its ability to be strongly attracted to a magnetic field and retain that magnetism. This property is fundamental to many of iron's applications.
The relatively low ionization energies of the 4s and 3d electrons explain iron's ability to form various oxidation states, commonly +2 and +3, contributing to its role in biological systems (hemoglobin) and its diverse chemical reactivity. Understanding its electronic structure is key to comprehending its ability to form various compounds and participate in oxidation-reduction reactions.
Applications of Understanding Electron Configuration
The knowledge of electron configuration isn't merely an academic exercise; it has significant practical applications:
-
Material Science: Understanding electron configurations is essential in developing new materials with desired properties. For example, it informs the design of alloys with specific magnetic, electrical, or catalytic characteristics.
-
Chemistry and Biochemistry: Predicting chemical reactivity and bonding relies heavily on understanding electron configurations. This is critical in various fields, including drug discovery, catalysis, and environmental chemistry.
-
Spectroscopy: Electron transitions between energy levels are responsible for the absorption and emission of light. Analyzing spectral data relies on a thorough understanding of electron configurations.
-
Nuclear Physics: Even though this discussion focuses on the electronic structure, understanding the arrangement of electrons informs us about the interactions between the electrons and the nucleus.
Conclusion
Drawing the electron configuration for a neutral iron atom, while seemingly a simple exercise, involves several fundamental principles of atomic structure. The detailed application of the Aufbau principle and Hund's rule, coupled with a visual representation through orbital diagrams, reveals a comprehensive picture of iron's electronic structure. This understanding is not just an academic endeavor; it is a cornerstone for numerous scientific and technological advancements across diverse fields. From the development of new materials to the understanding of biological processes, the knowledge of electron configuration remains indispensable.
Latest Posts
Latest Posts
-
60 Mph In Feet Per Second
Mar 25, 2025
-
What Is 5 6 As A Decimal
Mar 25, 2025
-
How Many Electrons In 3rd Energy Level
Mar 25, 2025
-
All Real Square Roots Of 4
Mar 25, 2025
-
Common Factors Of 36 And 24
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Draw The Electron Configuration For A Neutral Atom Of Iron. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
