Each Column In The Periodic Table Is Called A
listenit
Mar 23, 2025 · 6 min read
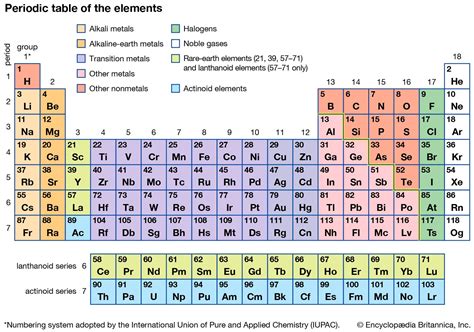
Table of Contents
Each Column in the Periodic Table is Called a Group: Exploring the Organization of Elements
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding its structure is crucial for grasping chemical behavior and predicting reactions. A common question that arises is: what is each column in the periodic table called? The answer is simple yet profound: each column in the periodic table is called a group, also sometimes referred to as a family. These groups represent elements with similar outer electron shell configurations, leading to shared chemical properties. This article will delve deep into the significance of groups, exploring their characteristics, trends, and importance in understanding the periodic system.
Understanding the Organization of the Periodic Table
Before delving into the specifics of groups, it's helpful to review the overall organization of the periodic table. Elements are arranged in order of increasing atomic number, which reflects the number of protons in the atom's nucleus. This arrangement isn't arbitrary; it's dictated by the underlying quantum mechanical principles governing electron configuration. The table is structured into rows (periods) and columns (groups).
Periods: Reflecting Electron Shells
The rows, or periods, represent the principal energy levels or electron shells. Elements within the same period have electrons filling the same principal energy level. As you move across a period from left to right, the number of electrons in the outermost shell (valence electrons) increases, resulting in a gradual change in properties.
Groups: Shared Electron Configurations and Properties
The columns, or groups, are where the real power of the periodic table lies. Elements within the same group share the same number of valence electrons, the electrons in the outermost shell that participate in chemical bonding. This shared electron configuration dictates similar chemical behavior and properties. This is why elements within the same group are often referred to as a family – they share a common ancestry, so to speak, in terms of their electronic structure.
The Significance of Group Numbering
Group numbering systems have evolved over time. Currently, two main numbering systems are used:
-
The IUPAC (International Union of Pure and Applied Chemistry) system: This system numbers the groups from 1 to 18, running from left to right across the periodic table. This system is the most widely accepted and used internationally.
-
The older American system: This system uses Roman numerals (IA, IIA, IIIA, etc.) for the representative elements (main group elements) and uses numbers for the transition elements (groups 3-12).
In this article, we will primarily utilize the IUPAC group numbering system for clarity and consistency.
Exploring Key Groups and their Characteristics
Let's explore some of the key groups and their characteristic properties:
Group 1: Alkali Metals (Li, Na, K, Rb, Cs, Fr)
Alkali metals are highly reactive metals with one valence electron. They readily lose this electron to form +1 ions, exhibiting low ionization energies and electronegativities. Their reactivity increases down the group. These elements are soft, silvery-white metals and react vigorously with water, producing hydrogen gas.
Group 2: Alkaline Earth Metals (Be, Mg, Ca, Sr, Ba, Ra)
Alkaline earth metals have two valence electrons and are less reactive than alkali metals. They form +2 ions and are harder and denser than alkali metals. They are also less reactive with water than alkali metals. Magnesium and calcium are biologically significant elements.
Group 17: Halogens (F, Cl, Br, I, At)
Halogens are highly reactive nonmetals with seven valence electrons. They readily gain one electron to form -1 ions, exhibiting high electron affinities and electronegativities. Their reactivity decreases down the group. Halogens are essential in various applications, including disinfectants and industrial chemicals.
Group 18: Noble Gases (He, Ne, Ar, Kr, Xe, Rn)
Noble gases have eight valence electrons (except helium, which has two), resulting in a stable electron configuration. This full valence shell makes them extremely unreactive, hence their name "noble gases." They are colorless, odorless, monatomic gases.
Group 14: Carbon Group (C, Si, Ge, Sn, Pb)
This group shows a fascinating diversity in properties. Carbon forms the basis of organic chemistry, while silicon is a crucial semiconductor material. The properties of elements in this group vary greatly due to the increasing metallic character down the group.
Transition Metals (Groups 3-12)
Transition metals exhibit variable oxidation states due to the involvement of d-electrons in bonding. This characteristic leads to a wide range of colorful compounds and catalytic properties. They are generally good conductors of electricity and heat. Many transition metals are essential in biological systems, playing crucial roles in enzymes and other biological processes.
Inner Transition Metals (Lanthanides and Actinides)
Located at the bottom of the periodic table, these elements have unique electronic configurations and properties. The lanthanides, also known as rare earth elements, are used extensively in modern technology. The actinides are mostly radioactive and include elements like uranium and plutonium.
Trends within Groups: Periodic Properties
The periodic table's brilliance lies in its ability to predict trends in properties within groups. Several key periodic properties exhibit predictable trends down a group:
-
Atomic Radius: Generally increases down a group due to the addition of electron shells.
-
Ionization Energy: Generally decreases down a group due to the increasing distance between the valence electrons and the nucleus.
-
Electronegativity: Generally decreases down a group due to the increasing atomic radius and shielding effect.
-
Metallic Character: Generally increases down a group, with elements at the bottom of a group tending to exhibit more metallic properties.
Understanding these trends is crucial for predicting the reactivity and chemical behavior of elements within a group.
The Importance of Groups in Chemistry and Beyond
The concept of groups is central to several aspects of chemistry and related fields:
-
Predicting Chemical Reactions: Understanding the group of an element provides insight into its likely reactivity and the types of chemical bonds it can form.
-
Material Science: The properties of elements within specific groups are exploited in material science to create materials with specific desired properties. For example, the use of semiconductors like silicon (Group 14) in electronics.
-
Biological Systems: Many elements from specific groups are essential for biological processes. For instance, the role of alkali metals and alkaline earth metals in maintaining electrolyte balance in the body.
-
Environmental Science: Understanding the reactivity and behavior of elements from specific groups is critical in environmental science, particularly in assessing the environmental impact of pollutants.
Conclusion: Groups – The Heart of the Periodic Table
Each column in the periodic table, a group or family, represents elements with shared electron configurations and, therefore, similar chemical properties. The periodic table's organization by groups is fundamental to understanding the relationships between elements, predicting chemical behavior, and advancing our knowledge in various scientific disciplines. The systematic arrangement of elements based on their electronic structure enables chemists and scientists to extrapolate information and make predictions about the behavior of elements, paving the way for numerous scientific advancements and technological breakthroughs. The groups, therefore, are not merely columns but the very foundation upon which the entire edifice of modern chemistry is built. Their study is crucial for anyone seeking to grasp the intricacies of the chemical world.
Latest Posts
Latest Posts
-
60 Mph In Feet Per Second
Mar 25, 2025
-
What Is 5 6 As A Decimal
Mar 25, 2025
-
How Many Electrons In 3rd Energy Level
Mar 25, 2025
-
All Real Square Roots Of 4
Mar 25, 2025
-
Common Factors Of 36 And 24
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Each Column In The Periodic Table Is Called A . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
