The Final Electron Acceptor Of Aerobic Cellular Respiration Is _____.
listenit
Mar 21, 2025 · 6 min read
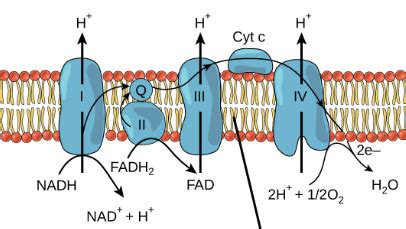
Table of Contents
The Final Electron Acceptor of Aerobic Cellular Respiration is Oxygen: A Deep Dive
The process of cellular respiration is the powerhouse of life, converting the energy stored in food molecules into a usable form of energy – ATP (adenosine triphosphate). This intricate process can be broadly classified into aerobic and anaerobic respiration, depending on the presence or absence of oxygen. The question, "The final electron acceptor of aerobic cellular respiration is _____," has a simple yet profound answer: oxygen. Understanding why oxygen holds this crucial role requires delving into the intricacies of the electron transport chain, the final stage of aerobic respiration.
Understanding Cellular Respiration: A Recap
Before we dive into the specifics of the final electron acceptor, let's briefly review the stages of aerobic cellular respiration. This metabolic pathway unfolds in three main stages:
-
Glycolysis: This initial stage occurs in the cytoplasm and breaks down glucose into two molecules of pyruvate, producing a small amount of ATP and NADH (nicotinamide adenine dinucleotide), an electron carrier. Glycolysis is anaerobic, meaning it doesn't require oxygen.
-
Krebs Cycle (Citric Acid Cycle): Pyruvate enters the mitochondria, where it's converted into acetyl-CoA, which then enters the Krebs cycle. This cycle generates ATP, NADH, FADH2 (flavin adenine dinucleotide), another electron carrier, and carbon dioxide as a waste product.
-
Electron Transport Chain (ETC) and Oxidative Phosphorylation: This is where the magic happens. The high-energy electrons carried by NADH and FADH2 are passed along a chain of protein complexes embedded in the inner mitochondrial membrane. This electron transfer releases energy, which is used to pump protons (H+) across the membrane, creating a proton gradient. This gradient drives ATP synthesis through chemiosmosis, generating the vast majority of ATP produced during cellular respiration. It is in this stage that oxygen plays its vital role as the final electron acceptor.
The Crucial Role of Oxygen: The Final Electron Acceptor
The electron transport chain is a carefully orchestrated series of redox reactions (reduction-oxidation reactions). Electrons are passed from one protein complex to the next, each step slightly lowering the energy level. If there were no final electron acceptor, the electron transport chain would come to a halt. The electrons would have nowhere to go, preventing further electron flow and ATP production. This is where oxygen steps in.
Oxygen's high electronegativity makes it an ideal final electron acceptor. This means it has a strong affinity for electrons. At the end of the electron transport chain, the electrons are finally transferred to oxygen molecules, along with protons (H+), forming water (H₂O). This reaction is crucial because it maintains the electron flow through the ETC, enabling the continuous pumping of protons and subsequent ATP synthesis. Without oxygen, the entire process grinds to a halt.
What Happens Without Oxygen? Anaerobic Respiration
In the absence of oxygen, the electron transport chain cannot function as described above. Organisms resort to anaerobic respiration, also known as fermentation. Fermentation is a less efficient process that produces far less ATP than aerobic respiration. Different organisms utilize different types of fermentation, such as lactic acid fermentation (in muscles during strenuous activity) or alcoholic fermentation (in yeast). These processes use alternative electron acceptors, such as pyruvate (in lactic acid fermentation) or acetaldehyde (in alcoholic fermentation). However, these alternative electron acceptors are less efficient than oxygen in terms of ATP production.
The consequences of insufficient oxygen can be profound. Without adequate oxygen, cells cannot produce sufficient ATP to meet their energy demands. This leads to a buildup of lactate or ethanol (depending on the type of fermentation), which can be toxic at high concentrations. In severe cases, oxygen deprivation can lead to cell death.
The Structure and Function of the Electron Transport Chain
The electron transport chain comprises four major protein complexes (Complex I-IV), as well as two mobile electron carriers, ubiquinone (CoQ) and cytochrome c. These components are embedded in the inner mitochondrial membrane, creating a highly organized system for electron transfer.
-
Complex I (NADH dehydrogenase): Accepts electrons from NADH.
-
Complex II (Succinate dehydrogenase): Accepts electrons from FADH2.
-
CoQ (Ubiquinone): A mobile electron carrier that transports electrons from Complex I and Complex II to Complex III.
-
Complex III (Cytochrome bc1 complex): Transfers electrons to cytochrome c.
-
Cytochrome c: Another mobile electron carrier that transports electrons from Complex III to Complex IV.
-
Complex IV (Cytochrome c oxidase): The final complex, which transfers electrons to oxygen, forming water.
The precise mechanism of electron transfer involves a complex interplay of redox reactions, proton pumping, and conformational changes within the protein complexes. This intricate system ensures highly efficient energy conversion.
Oxygen's Role in Maintaining the Proton Gradient
The proton gradient generated across the inner mitochondrial membrane is absolutely crucial for ATP synthesis. The energy released during electron transfer is directly used to pump protons from the mitochondrial matrix into the intermembrane space. This creates a higher concentration of protons in the intermembrane space, generating a proton motive force (PMF). This PMF drives ATP synthesis through ATP synthase, an enzyme that acts as a channel for protons to flow back into the matrix. The flow of protons through ATP synthase drives the rotation of a part of the enzyme, leading to the synthesis of ATP from ADP and inorganic phosphate (Pi).
Oxygen's role as the final electron acceptor is essential for maintaining this proton gradient. Without oxygen to accept the electrons, the electron transport chain would cease to function, leading to the collapse of the proton gradient and consequently a significant reduction in ATP synthesis.
Oxygen Toxicity and Reactive Oxygen Species (ROS)
While oxygen is essential for aerobic respiration, it also poses a risk. During electron transport, a small percentage of electrons can leak out of the chain and react with oxygen to form reactive oxygen species (ROS), such as superoxide radicals (O₂⁻) and hydrogen peroxide (H₂O₂). These ROS are highly reactive and can damage cellular components, including DNA, proteins, and lipids, leading to oxidative stress.
Cells have developed various defense mechanisms to combat ROS, including enzymes like superoxide dismutase (SOD) and catalase, which convert ROS into less harmful molecules. However, excessive ROS production can overwhelm these defense mechanisms, contributing to aging and various diseases.
Alternative Electron Acceptors: A Glimpse into Anaerobic Life
Although oxygen is the most efficient final electron acceptor, some organisms have evolved to utilize alternative electron acceptors under anaerobic conditions. These alternative acceptors can include nitrate (NO₃⁻), sulfate (SO₄²⁻), or even carbon dioxide (CO₂). These processes are less efficient than aerobic respiration, producing less ATP, but they allow these organisms to thrive in environments lacking oxygen.
These anaerobic respiration pathways are critical for the biogeochemical cycles of various elements like nitrogen and sulfur. They play vital roles in various ecosystems, influencing nutrient cycling and overall ecosystem health. Understanding these alternative electron acceptors is crucial for comprehending the diversity of life on Earth and the adaptability of organisms to various environmental conditions.
Conclusion: The Indispensable Role of Oxygen
In summary, the final electron acceptor of aerobic cellular respiration is oxygen. This crucial role is multifaceted, underpinning the efficiency of the electron transport chain, maintaining the proton gradient necessary for ATP synthesis, and ultimately powering the vast majority of life on Earth. While alternative electron acceptors exist for anaerobic organisms, oxygen's high electronegativity makes it the most efficient and vital final electron acceptor for aerobic respiration, emphasizing its pivotal role in the energy metabolism of countless organisms. Understanding this fundamental process is essential for comprehending cellular biology and the intricate mechanisms that drive life.
Latest Posts
Latest Posts
-
What Percentage Is 7 Of 12
Mar 28, 2025
-
Lowest Common Factor Of 7 And 9
Mar 28, 2025
-
2 Protons 2 Neutrons 2 Electrons
Mar 28, 2025
-
How Many Electrons Can The P Orbital Hold
Mar 28, 2025
-
What Is Another Name For Newtons First Law
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about The Final Electron Acceptor Of Aerobic Cellular Respiration Is _____. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
