How Many Electrons Can The P Orbital Hold
listenit
Mar 28, 2025 · 6 min read
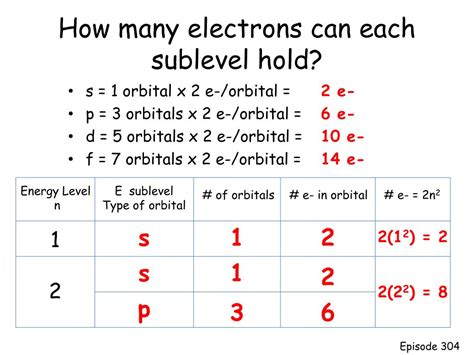
Table of Contents
How Many Electrons Can the p Orbital Hold? A Deep Dive into Atomic Structure
Understanding the structure of atoms is fundamental to comprehending chemistry and physics. A key aspect of this understanding involves electron configuration and the orbitals that house these electrons. One frequently asked question revolves around the p orbital: how many electrons can a p orbital hold? This article will delve into the answer, exploring the quantum mechanical principles that govern electron behavior and explaining the significance of this capacity in determining an atom's properties.
Understanding Atomic Orbitals
Before addressing the capacity of the p orbital, let's establish a basic understanding of atomic orbitals. According to the quantum mechanical model of the atom, electrons don't orbit the nucleus in neat, predictable paths like planets around the sun. Instead, they exist in regions of space called orbitals, which are described by wave functions. These wave functions define the probability of finding an electron at a particular location.
Orbitals are categorized into different types based on their energy levels and shapes:
-
s orbitals: These are spherical in shape, with the nucleus at the center. Each energy level has one s orbital.
-
p orbitals: These are dumbbell-shaped, with two lobes on either side of the nucleus. Each energy level (starting from the second) has three p orbitals, oriented along the x, y, and z axes. They are often designated as p<sub>x</sub>, p<sub>y</sub>, and p<sub>z</sub>.
-
d orbitals: These are more complex in shape, with four of the five d orbitals having a cloverleaf-like appearance. They appear from the third energy level onwards.
-
f orbitals: These orbitals possess even more intricate shapes and appear from the fourth energy level onwards.
The Pauli Exclusion Principle: A Key to Electron Capacity
The key to determining how many electrons a p orbital (or any orbital) can hold lies in the Pauli Exclusion Principle. This fundamental principle of quantum mechanics states that no two electrons in an atom can have the same set of four quantum numbers. These quantum numbers describe the properties of an electron within an atom:
-
Principal quantum number (n): Describes the energy level of the electron (n = 1, 2, 3...).
-
Azimuthal quantum number (l): Describes the shape of the orbital (l = 0 for s, l = 1 for p, l = 2 for d, l = 3 for f...).
-
Magnetic quantum number (m<sub>l</sub>): Describes the orientation of the orbital in space (m<sub>l</sub> = -l, ..., 0, ..., +l). For p orbitals (l=1), m<sub>l</sub> can be -1, 0, or +1, corresponding to the p<sub>x</sub>, p<sub>y</sub>, and p<sub>z</sub> orbitals.
-
Spin quantum number (m<sub>s</sub>): Describes the intrinsic angular momentum of the electron, often referred to as "spin." It can have only two values: +1/2 (spin up) or -1/2 (spin down).
Because each orbital is defined by a specific combination of n, l, and m<sub>l</sub>, the Pauli Exclusion Principle limits the number of electrons in any given orbital to two: one with spin up (+1/2) and one with spin down (-1/2).
How Many Electrons in a p Sublevel?
It's crucial to differentiate between a p orbital and a p sublevel. A p sublevel comprises the three p orbitals (p<sub>x</sub>, p<sub>y</sub>, and p<sub>z</sub>) that exist within a given energy level. Since each p orbital can hold two electrons, and there are three p orbitals in a p sublevel, a p sublevel can hold a total of six electrons.
This is a significant point for understanding electron configurations. For example, in the element phosphorus (P), the electron configuration is 1s²2s²2p⁶3s²3p³. The 2p sublevel is completely filled with six electrons, while the 3p sublevel contains three electrons.
The Importance of Electron Configuration and Orbital Filling
Understanding how many electrons a p orbital can hold is crucial for predicting an element's chemical behavior. The arrangement of electrons in orbitals, known as electron configuration, directly influences the atom's reactivity and bonding properties. The outermost electrons, those in the highest energy level, are particularly important in this regard, as they participate in chemical reactions.
Elements with completely filled p sublevels (containing six electrons) are often less reactive than elements with partially filled p sublevels. This is because filled sublevels represent a stable electron configuration, and atoms tend to seek this stability through chemical bonding.
Exceptions to the Rules: Hund's Rule
While the Pauli Exclusion Principle dictates that each orbital can hold a maximum of two electrons, Hund's Rule provides guidance on how electrons fill orbitals within a sublevel. Hund's Rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This is because electrons repel each other and prefer to be in separate orbitals as much as possible.
For instance, in a nitrogen atom (electron configuration 1s²2s²2p³), each of the three 2p orbitals will be occupied by a single electron before any orbital receives a second electron. Only then will electrons start pairing up within the orbitals.
Beyond the Basics: Orbital Hybridization and Molecular Orbitals
The concepts discussed so far relate primarily to atomic orbitals. When atoms bond to form molecules, their atomic orbitals combine to form molecular orbitals. This process, known as orbital hybridization, can lead to changes in the shapes and energies of the orbitals, influencing molecular geometry and reactivity.
Understanding the basic capacity of atomic orbitals, however, remains essential even when considering the complexities of molecular orbital theory. The principles of quantum mechanics, including the Pauli Exclusion Principle, provide the foundation upon which our understanding of chemical bonding and molecular structure is built.
Conclusion: The Significance of Electron Capacity in the P Orbital
In summary, a single p orbital can hold a maximum of two electrons, one with spin up and one with spin down, as dictated by the Pauli Exclusion Principle. A p sublevel, however, consisting of three p orbitals, can accommodate up to six electrons. This seemingly simple fact is fundamental to our understanding of atomic structure, electron configuration, chemical bonding, and the properties of matter. The capacity of the p orbital, along with other orbital filling rules, determines the reactivity and behavior of elements and forms the bedrock of modern chemistry. Further exploration of quantum mechanics and its applications reveals even more complex and fascinating aspects of electron behavior within atoms and molecules. Understanding this fundamental principle is essential for anyone seeking a deeper understanding of the world at the atomic level.
Latest Posts
Latest Posts
-
What Is The Percentage Of 4 Out Of 20
Mar 31, 2025
-
How To Find The Restricted Domain
Mar 31, 2025
-
What Is 1 3 4 In Decimal Form
Mar 31, 2025
-
What Is The Derivative Of X 5
Mar 31, 2025
-
How Many Even Numbers Are On A Dice
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can The P Orbital Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
