The Change Of State From Liquid To Gas Is Called
listenit
Apr 01, 2025 · 6 min read
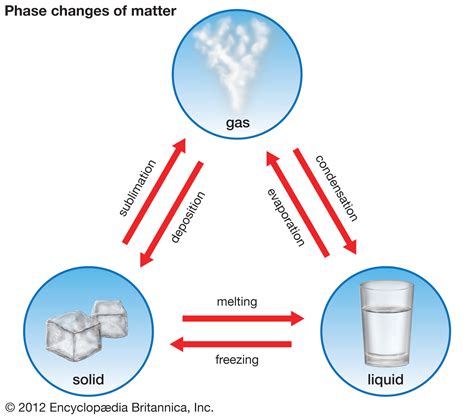
Table of Contents
The Change of State from Liquid to Gas is Called Vaporization: A Deep Dive
The change of state from liquid to gas is called vaporization. This seemingly simple process is a fundamental concept in chemistry and physics, underpinning countless natural phenomena and industrial applications. Understanding vaporization requires exploring its various forms, the factors influencing it, and its significant role in our world. This comprehensive guide will delve into the intricacies of vaporization, examining its mechanisms, applications, and real-world implications.
Understanding Vaporization: More Than Just Boiling
Vaporization, broadly defined, is the phase transition where a substance in its liquid state transforms into a gaseous state. This transformation isn't a uniform process; rather, it encompasses several distinct mechanisms, each characterized by specific conditions and observable characteristics.
1. Evaporation: A Gentle Transition
Evaporation is the gradual transformation of a liquid into a gas that occurs at temperatures below the liquid's boiling point. It's a surface phenomenon, where molecules with sufficient kinetic energy escape from the liquid's surface, overcoming the intermolecular forces holding them together. Think of a puddle drying on a sunny day – this is evaporation in action. The rate of evaporation depends on several factors, including:
- Temperature: Higher temperatures mean more molecules possess the energy to escape.
- Surface area: A larger surface area exposes more molecules to the atmosphere, accelerating the process.
- Humidity: High humidity (high concentration of water vapor in the air) reduces evaporation as the air is already saturated.
- Airflow: Moving air removes vapor molecules from the surface, allowing more to escape.
Keywords: evaporation, surface phenomenon, kinetic energy, intermolecular forces, temperature, surface area, humidity, airflow.
2. Boiling: A Violent Transformation
Boiling, unlike evaporation, is a bulk phenomenon occurring throughout the liquid. It happens when the liquid's vapor pressure equals the external pressure (usually atmospheric pressure). At this point, bubbles of vapor form within the liquid, rising to the surface and releasing the gas. The temperature at which boiling occurs is the boiling point, a characteristic property of each substance. The boiling point is affected by:
- Pressure: Lower external pressure leads to a lower boiling point. This is why water boils at a lower temperature at high altitudes where atmospheric pressure is lower.
- Impurities: Dissolved impurities can slightly elevate the boiling point.
Keywords: boiling, bulk phenomenon, vapor pressure, external pressure, atmospheric pressure, boiling point, pressure, impurities.
3. Sublimation (A Special Case): Solid to Gas
While not strictly a liquid-to-gas transition, sublimation is a related phase change worthy of mention. Sublimation involves the direct transformation of a solid into a gas, bypassing the liquid phase entirely. This occurs when the vapor pressure of the solid exceeds the external pressure. Dry ice (solid carbon dioxide) is a common example; it sublimates directly into carbon dioxide gas at room temperature.
Keywords: sublimation, solid to gas, vapor pressure, external pressure, dry ice, carbon dioxide.
Factors Influencing Vaporization: A Deeper Look
Several factors beyond temperature and pressure significantly influence the rate and extent of vaporization. These include:
-
Intermolecular Forces: Stronger intermolecular forces (like hydrogen bonds in water) require more energy to overcome, resulting in lower vaporization rates. Substances with weaker intermolecular forces vaporize more readily.
-
Molar Mass: Heavier molecules tend to have lower vapor pressures and thus vaporize more slowly than lighter molecules.
-
Heat of Vaporization: This is the amount of heat energy required to vaporize one mole of a liquid at its boiling point. Substances with high heats of vaporization require considerable energy input for vaporization.
Applications of Vaporization: From Everyday Life to Industry
Vaporization is a fundamental process with numerous applications across diverse fields:
-
Cooling Systems: Evaporation's cooling effect is exploited in sweat and refrigeration systems. As liquids evaporate, they absorb heat from their surroundings, leading to cooling.
-
Distillation: This process separates liquids with different boiling points based on their vaporization characteristics. It's crucial in various industries, including petroleum refining and the production of alcoholic beverages.
-
Spray Drying: This technique uses vaporization to produce dry powders from liquid solutions. It's widely used in the food industry (e.g., milk powder) and pharmaceutical manufacturing.
-
Power Generation: Vaporization is central to steam power plants, where water is vaporized to drive turbines and generate electricity.
-
Atmospheric Processes: The water cycle relies heavily on evaporation and condensation (the reverse of vaporization). These processes are essential for weather patterns, precipitation, and climate regulation.
The Role of Vapor Pressure: A Key Parameter
Vapor pressure is a critical concept in understanding vaporization. It refers to the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (liquid or solid) at a given temperature. A higher vapor pressure indicates a greater tendency for a substance to vaporize. The vapor pressure increases with temperature, explaining why substances vaporize more readily at higher temperatures.
Vaporization and the Clausius-Clapeyron Equation
The relationship between vapor pressure and temperature is described by the Clausius-Clapeyron equation, a powerful tool for predicting vapor pressure at different temperatures. This equation is crucial in various applications, including designing distillation columns and predicting the behavior of substances under different conditions. The equation relates the natural logarithm of vapor pressure to the reciprocal of temperature, involving the heat of vaporization and a constant.
Real-World Examples and Implications: Seeing Vaporization in Action
Understanding vaporization extends beyond textbook definitions; it's vital in interpreting everyday phenomena and solving real-world problems.
-
Drying Clothes: The sun's heat accelerates evaporation, helping clothes dry faster.
-
Cooking: Boiling water for pasta or steaming vegetables utilizes vaporization.
-
Humidity and Comfort: High humidity slows evaporation from our skin, making us feel warmer and stickier.
-
Cloud Formation: Water vapor in the atmosphere condenses to form clouds, a crucial element of the water cycle.
-
Climate Change: Increased evaporation due to rising global temperatures can exacerbate weather extremes and impact water resources.
Conclusion: A Fundamental Process with Far-Reaching Consequences
The change of state from liquid to gas, or vaporization, is far from a simple process. Its various forms, influencing factors, and widespread applications underscore its importance in chemistry, physics, and numerous engineering disciplines. From everyday occurrences like drying clothes to industrial processes like distillation and power generation, vaporization plays a fundamental role in shaping our world. A comprehensive understanding of vaporization, its mechanisms, and the factors that govern it, is essential for both scientific advancement and technological innovation. Furthermore, appreciating the role of vaporization in atmospheric processes and its implications for climate change highlights the importance of this fundamental phase transition in our understanding of the planet's complex systems. By grasping the intricate details of vaporization, we gain a deeper understanding of the physical world around us.
Latest Posts
Latest Posts
-
What Is The Most Reactive Group On The Periodic Table
Apr 02, 2025
-
What Is 3 Divided By 11
Apr 02, 2025
-
How To Find The Mass Of The Excess Reactant
Apr 02, 2025
-
Instantaneous Rate Of Change Vs Average Rate Of Change
Apr 02, 2025
-
How Many D Orbitals Can Be In An Energy Level
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about The Change Of State From Liquid To Gas Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
