Number Of Valence Electrons In Aluminum
listenit
Mar 21, 2025 · 5 min read
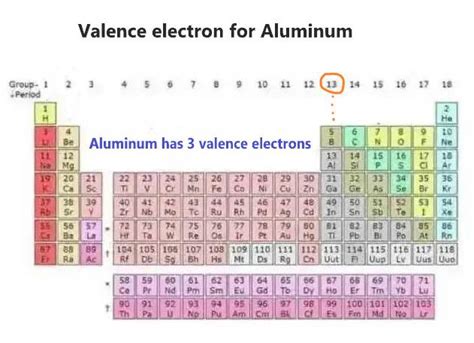
Table of Contents
Unveiling the Mysteries of Aluminum: Delving into its Valence Electrons
Aluminum, a ubiquitous metal found in everything from soda cans to airplanes, holds a fascinating place in the world of chemistry. Understanding its properties, particularly its valence electrons, is key to comprehending its reactivity and diverse applications. This in-depth exploration will delve into the number of valence electrons in aluminum, explaining its significance and how it impacts its chemical behavior.
What are Valence Electrons?
Before we pinpoint the number of valence electrons in aluminum, let's establish a firm understanding of what valence electrons actually are. Valence electrons are the electrons located in the outermost shell, or energy level, of an atom. These electrons are crucial because they determine an element's chemical properties and how it will interact with other atoms. They're the primary participants in chemical bonding, influencing an element's reactivity, the types of bonds it forms (ionic, covalent, metallic), and the number of bonds it can create. Think of them as the atom's "social butterflies"—the ones that interact most frequently with the outside world.
Determining Aluminum's Electronic Configuration
To find the number of valence electrons in aluminum, we need to examine its electronic configuration. This configuration describes how electrons are distributed among the various energy levels and sublevels within an atom. Aluminum's atomic number is 13, meaning it has 13 protons and 13 electrons in a neutral atom.
Using the Aufbau principle and Hund's rule, we can determine the electronic configuration of aluminum: 1s²2s²2p⁶3s²3p¹. This notation tells us:
- 1s²: Two electrons occupy the first energy level (n=1) in the 's' subshell.
- 2s²: Two electrons occupy the second energy level (n=2) in the 's' subshell.
- 2p⁶: Six electrons occupy the second energy level (n=2) in the 'p' subshell.
- 3s²: Two electrons occupy the third energy level (n=3) in the 's' subshell.
- 3p¹: One electron occupies the third energy level (n=3) in the 'p' subshell.
Identifying the Valence Electrons in Aluminum
The valence electrons are those in the outermost energy level. In aluminum's case, the outermost energy level is the third energy level (n=3). Therefore, we add the electrons in the 3s and 3p subshells: 2 (from 3s²) + 1 (from 3p¹) = 3 valence electrons.
Therefore, aluminum possesses 3 valence electrons. This seemingly small number has significant implications for aluminum's chemical behavior.
The Significance of Three Valence Electrons
The presence of three valence electrons profoundly influences aluminum's properties and reactivity:
1. Metallic Bonding and Conductivity:
Aluminum's three valence electrons are relatively loosely held. This allows them to be easily delocalized, forming a "sea" of electrons surrounding the positively charged aluminum nuclei. This is the essence of metallic bonding. The free movement of these delocalized electrons is what accounts for aluminum's excellent electrical and thermal conductivity. The electrons can readily carry charge and energy throughout the metal structure.
2. Reactivity and Oxidation:
Aluminum's three valence electrons readily participate in chemical reactions. Aluminum tends to lose these three electrons to achieve a stable octet configuration, resembling the noble gas neon. This electron loss results in the formation of the Al³⁺ ion, a process known as oxidation. This explains why aluminum is relatively reactive, although it's protected by a thin, tenacious layer of aluminum oxide (Al₂O₃) that forms readily upon exposure to air, preventing further oxidation and corrosion.
3. Formation of Compounds:
Aluminum's three valence electrons allow it to form a variety of compounds. It readily forms ionic bonds with nonmetals by transferring its three valence electrons to achieve a stable electron configuration. For example, aluminum chloride (AlCl₃) is formed when aluminum loses three electrons to three chlorine atoms, each of which gains one electron. Aluminum can also participate in covalent bonding, although less frequently than ionic bonding.
4. Alloy Formation:
Aluminum's ability to form metallic bonds with other metals contributes to its widespread use in alloys. Aluminum alloys are known for their strength, lightweight nature, and corrosion resistance. Adding other elements to aluminum alters its properties, enhancing its strength, ductility, or other desirable characteristics.
Aluminum's Applications: A Testament to its Valence Electrons
The unique properties stemming from its three valence electrons make aluminum incredibly versatile and suitable for a wide range of applications:
- Packaging: Aluminum's malleability, corrosion resistance, and light weight make it ideal for food and beverage packaging (cans, foil).
- Transportation: Its strength-to-weight ratio is crucial in aircraft construction, automotive parts, and train carriages.
- Construction: Aluminum is used extensively in building materials, offering durability and lightweight construction.
- Electrical Engineering: Its high electrical conductivity makes it essential in power transmission lines and electrical components.
- Consumer Electronics: Aluminum finds its way into smartphones, laptops, and other electronic devices.
Comparing Aluminum's Valence Electrons to Other Elements
Comparing aluminum's three valence electrons to other elements in the periodic table further highlights its unique characteristics:
- Group 1 Elements (Alkali Metals): These elements have only one valence electron and are highly reactive, readily losing that electron to form +1 ions. Aluminum, with three valence electrons, is less reactive than alkali metals because it requires more energy to lose three electrons.
- Group 2 Elements (Alkaline Earth Metals): These have two valence electrons and are less reactive than alkali metals but still more reactive than aluminum.
- Group 13 Elements (Boron Group): Aluminum belongs to this group. Other elements in this group, like boron, also have three valence electrons, but their properties differ due to variations in atomic size and other factors.
- Transition Metals: Transition metals have variable valence electrons, often exhibiting multiple oxidation states. Aluminum, with its consistently three valence electrons, is simpler in this regard.
Conclusion: The Importance of Understanding Valence Electrons
Understanding the number of valence electrons in aluminum – three – is crucial for grasping its chemical behavior and explaining its diverse applications. This seemingly simple number dictates its metallic bonding, reactivity, ability to form compounds and alloys, and ultimately, its widespread use in various industries. This detailed exploration underscores the fundamental importance of electronic configuration in determining the properties and behavior of elements and their place in the wider world of chemistry and materials science. The three valence electrons of aluminum are not just numbers; they are the key to unlocking the potential of this remarkably versatile metal.
Latest Posts
Latest Posts
-
What Are The Most Reactive Metals In The Periodic Table
Mar 28, 2025
-
Is Supports Combustion A Physical Or Chemical Property
Mar 28, 2025
-
What Is 6 Divided By 1 3
Mar 28, 2025
-
Add Acid To Water Or Water To Acid
Mar 28, 2025
-
How Many Calories Are In One Gram Of Lipids
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Aluminum . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
