Number Of Protons Neutrons And Electrons In Carbon
listenit
Mar 31, 2025 · 5 min read
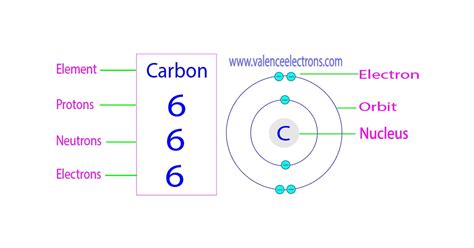
Table of Contents
Delving Deep into Carbon: Protons, Neutrons, and Electrons
Carbon, the cornerstone of life and a fundamental element in the universe, boasts a fascinating atomic structure. Understanding the number of protons, neutrons, and electrons in a carbon atom is crucial to comprehending its chemical properties and its vital role in countless processes. This comprehensive article will explore the specifics of carbon's atomic composition, delve into isotopic variations, and discuss the implications of this elemental makeup on its reactivity and importance.
Understanding the Basics of Atomic Structure
Before diving into the specifics of carbon, let's establish a foundational understanding of atomic structure. An atom, the fundamental unit of matter, consists of three primary subatomic particles:
- Protons: Positively charged particles residing within the atom's nucleus. The number of protons defines the element; it's the atomic number.
- Neutrons: Neutral particles (no charge) also located in the nucleus. Neutrons contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
The arrangement of these particles dictates an element's chemical behavior and its interactions with other atoms.
The Atomic Structure of Carbon: Protons, Neutrons, and Electrons
Carbon, represented by the symbol "C" and having an atomic number of 6, possesses:
- 6 Protons: This defining characteristic makes it carbon. The positive charge of these protons is balanced by...
- 6 Electrons: In its neutral state, a carbon atom has 6 electrons orbiting its nucleus, typically filling the first two shells (2 electrons in the first shell, 4 in the second). This electron configuration drives carbon's ability to form four covalent bonds, a property crucial to its role in organic chemistry.
- Variable Number of Neutrons: This is where things get interesting. The number of neutrons in a carbon atom can vary, leading to different isotopes.
Carbon Isotopes: Variations in Neutron Number
Isotopes are atoms of the same element with the same number of protons but differing numbers of neutrons. This difference in neutron number affects the atom's mass but not its chemical properties significantly. Carbon has three naturally occurring isotopes:
-
Carbon-12 (¹²C): This is the most abundant isotope, comprising approximately 98.9% of naturally occurring carbon. It contains 6 protons and 6 neutrons. It's the standard against which atomic mass units (amu) are defined.
-
Carbon-13 (¹³C): A less abundant stable isotope (around 1.1%), it has 6 protons and 7 neutrons. Its slightly heavier mass makes it useful in various scientific applications, including radiocarbon dating and nuclear magnetic resonance (NMR) spectroscopy.
-
Carbon-14 (¹⁴C): This is a radioactive isotope with 6 protons and 8 neutrons. It's formed in the upper atmosphere through cosmic ray interactions and decays with a half-life of approximately 5,730 years. This radioactive decay is the basis for radiocarbon dating, a technique used to determine the age of organic materials.
The Significance of Carbon's Atomic Structure
The specific arrangement of protons, neutrons, and electrons in a carbon atom underlies its exceptional importance in various fields:
1. Organic Chemistry and the Basis of Life
Carbon's ability to form four covalent bonds is unparalleled. This tetravalency allows carbon atoms to link together to form long chains, branched structures, and rings, creating the immense diversity of organic molecules that underpin all known life forms. From simple carbohydrates and lipids to complex proteins and nucleic acids (DNA and RNA), carbon's versatility is fundamental to the structure and function of living organisms.
2. Materials Science and Industrial Applications
Carbon's unique properties lead to diverse applications in materials science and various industries. The allotropes of carbon—different structural forms of the same element—exhibit significantly varying characteristics:
-
Diamond: A crystalline allotrope known for its exceptional hardness, refractive index, and thermal conductivity. It finds use in cutting tools, jewelry, and high-tech applications.
-
Graphite: A layered allotrope known for its softness, lubricity, and electrical conductivity. It's used in pencils, lubricants, and electrodes.
-
Fullerenes (e.g., Buckminsterfullerene or Buckyballs): Spherical or ellipsoidal molecules composed of carbon atoms arranged in a cage-like structure. Their unique properties are being explored for applications in nanotechnology and medicine.
-
Graphene: A single layer of carbon atoms arranged in a hexagonal lattice. It's known for its exceptional strength, electrical conductivity, and thermal conductivity, making it a material of immense potential in electronics, composites, and energy storage.
3. Nuclear Science and Radiocarbon Dating
Carbon-14's radioactive decay is a cornerstone of radiocarbon dating. By measuring the ratio of ¹⁴C to ¹²C in organic materials, scientists can estimate the time elapsed since the organism died. This technique is invaluable in archaeology, geology, and paleontology for dating artifacts, fossils, and other ancient materials.
4. Medical Imaging and Spectroscopy
Carbon-13's unique nuclear spin properties make it useful in nuclear magnetic resonance (NMR) spectroscopy and magnetic resonance imaging (MRI). These techniques are crucial for studying the structure and function of molecules and tissues in medicine and materials science.
Conclusion: The Importance of Understanding Carbon's Atomic Structure
The seemingly simple atomic structure of carbon—with its 6 protons, 6 electrons, and variable number of neutrons—underlies its extraordinary importance in the universe. Understanding the number and arrangement of these subatomic particles is key to grasping carbon's chemical reactivity, its diverse allotropic forms, and its essential role in life, materials science, and various scientific applications. From the building blocks of life to high-tech materials and archaeological dating, the profound impact of carbon stems directly from the fundamental characteristics of its atomic structure. Further research into carbon's properties and potential applications continues to reveal its ever-expanding significance in our world. The study of its isotopes and their varied applications underscores the ongoing relevance of understanding the nuances of its atomic composition. The fundamental principles explored here serve as a gateway to more complex concepts in chemistry, physics, and related fields. This knowledge empowers us to comprehend the intricate workings of the natural world and to harness the potential of this remarkable element for the betterment of humanity.
Latest Posts
Latest Posts
-
Is Koh A Base Or Acid
Apr 02, 2025
-
Why Did Small States Object To The Virginia Plan
Apr 02, 2025
-
What Is The Proper Name For Mgf2
Apr 02, 2025
-
Unit Of Measurement For Kinetic Energy
Apr 02, 2025
-
How Do Lichens Contribute To Primary Succession
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Number Of Protons Neutrons And Electrons In Carbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
