Number Of Electrons In Carbon -14
listenit
Mar 24, 2025 · 5 min read
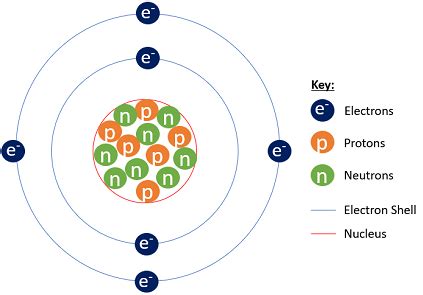
Table of Contents
The Number of Electrons in Carbon-14: A Deep Dive into Isotopes and Atomic Structure
Carbon-14, a fascinating isotope of the ubiquitous element carbon, plays a crucial role in various scientific fields, notably in radiocarbon dating. Understanding its atomic structure, particularly the number of electrons it possesses, is fundamental to grasping its properties and applications. This article delves into the details of carbon-14, exploring its electron count, isotopic variations, and significance in scientific advancements.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before focusing on carbon-14 specifically, let's establish a foundational understanding of atomic structure. Atoms, the fundamental building blocks of matter, consist of three primary subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines an element's atomic number and its identity.
- Neutrons: Neutrally charged particles also residing in the nucleus. Neutrons contribute to an atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom, ensuring overall electrical neutrality.
The arrangement of electrons in shells dictates an atom's chemical behavior and its ability to form bonds with other atoms.
Isotopes: Variations in Neutron Number
Isotopes are atoms of the same element (same number of protons) that differ in their number of neutrons. This variation in neutron count affects the atom's mass but not its chemical properties significantly. Carbon, for example, has three naturally occurring isotopes:
- Carbon-12 (¹²C): The most abundant isotope, containing 6 protons and 6 neutrons.
- Carbon-13 (¹³C): A stable isotope with 6 protons and 7 neutrons.
- Carbon-14 (¹⁴C): A radioactive isotope with 6 protons and 8 neutrons.
It's the difference in neutron number that distinguishes these isotopes. Their chemical behavior, however, remains largely similar because the number of protons (and therefore electrons) is identical.
The Number of Electrons in Carbon-14: A Simple Answer
The key takeaway is that a neutral carbon-14 atom has 6 electrons. This is because the atomic number of carbon is 6, indicating 6 protons in its nucleus. In a neutral atom, the number of electrons always equals the number of protons to balance the positive and negative charges. The extra neutrons in carbon-14 only affect its mass and radioactive properties, not its electron count.
Radioactive Decay of Carbon-14: A Closer Look
Carbon-14's radioactivity stems from its unstable nucleus, containing an excess of neutrons. This instability leads to beta decay, a process where a neutron transforms into a proton, emitting a beta particle (an electron) and an antineutrino. This transformation increases the atomic number by one, converting carbon-14 into nitrogen-14 (¹⁴N).
This radioactive decay is a first-order process, meaning the rate of decay is proportional to the amount of carbon-14 present. The half-life of carbon-14 is approximately 5,730 years, meaning half of a given sample will decay into nitrogen-14 within this timeframe. This predictable decay rate forms the basis of radiocarbon dating.
Radiocarbon Dating: Utilizing Carbon-14's Decay
The application of carbon-14's radioactive decay is revolutionary in archaeology and other fields. Living organisms continuously exchange carbon with their environment, maintaining a relatively constant ratio of ¹⁴C to ¹²C. However, upon death, this exchange ceases. The ¹⁴C in the organism begins to decay without replenishment. By measuring the remaining ¹⁴C in a sample and comparing it to the initial ¹⁴C ratio, scientists can estimate the time elapsed since the organism's death. This technique is invaluable in dating ancient artifacts, fossils, and other organic materials, pushing back the boundaries of our understanding of history and prehistory.
Beyond Radiocarbon Dating: Other Applications of Carbon-14
While radiocarbon dating is the most well-known application, carbon-14 finds its use in various scientific areas:
- Tracing Biochemical Processes: Researchers use carbon-14 as a tracer to study metabolic pathways and the movement of molecules within living organisms. Its radioactive nature enables tracking and quantification of specific molecules or processes.
- Climate Change Research: Analysis of ¹⁴C levels in ice cores, tree rings, and other environmental samples provides insights into past climate variations and atmospheric changes.
- Environmental Studies: Carbon-14 tracing is employed to track pollutants in the environment, enabling scientists to understand their dispersal patterns and impact on ecosystems.
- Medical Research: Although less common than other isotopes, carbon-14 has applications in medical research, particularly in drug development and metabolic studies.
The versatility of carbon-14 stems from its unique properties: readily incorporated into biological molecules and its predictable decay rate.
The Significance of Isotopic Abundance
The abundance of each carbon isotope in nature significantly influences the results of radiocarbon dating and other applications. The relatively low abundance of ¹⁴C (around 1 part per trillion in the atmosphere) necessitates sensitive measurement techniques. Variations in ¹⁴C abundance over time, due to factors like solar activity and cosmic ray flux, require careful calibration of radiocarbon dating results.
Understanding isotopic abundance is crucial for accurate interpretations and reliable conclusions in various scientific disciplines.
Conclusion: The Importance of Understanding Carbon-14
In summary, a neutral carbon-14 atom possesses 6 electrons, mirroring its 6 protons. While the extra neutrons distinguish it as an isotope, its chemical behavior remains similar to other carbon isotopes. Carbon-14's radioactive decay, with its characteristic half-life, has revolutionized archaeology and paleontology through radiocarbon dating. Moreover, its applications extend to numerous fields, including biochemistry, climate science, and environmental research. Comprehending the number of electrons in carbon-14, along with its isotopic variations and decay characteristics, is essential for appreciating its significance in various scientific advancements and our understanding of the world around us. Continued research into carbon-14's properties and applications promises further breakthroughs across various disciplines. Further exploration of its behaviour under varying conditions remains a key area for future scientific inquiry, enhancing our tools for investigating past environments and processes. The ongoing refinement of radiocarbon dating techniques, coupled with a deeper understanding of isotopic variations and their implications, ensures its continued relevance in scientific research for years to come.
Latest Posts
Latest Posts
-
What Is Half A Mile In Feet
Mar 26, 2025
-
What Percent Of 12 5 Is 39
Mar 26, 2025
-
Empirical And Molecular Formula Of Ibuprofen
Mar 26, 2025
-
What Is The Molar Mass Of So2
Mar 26, 2025
-
Electrons In The Outermost Energy Level Are Called
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Number Of Electrons In Carbon -14 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
