Net Ionic Equation For Hcl And Naoh
listenit
Mar 19, 2025 · 5 min read
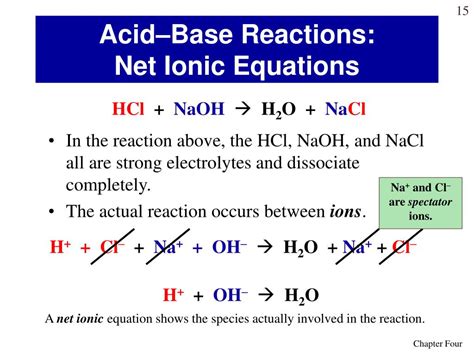
Table of Contents
Net Ionic Equation for HCl and NaOH: A Deep Dive into Acid-Base Reactions
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic example of a strong acid-strong base neutralization reaction. Understanding this reaction, particularly its net ionic equation, is fundamental to grasping the concepts of acid-base chemistry and solution stoichiometry. This article will provide a comprehensive explanation of this reaction, exploring its balanced molecular equation, complete ionic equation, and finally, the crucial net ionic equation. We will also delve into the underlying principles and applications of this reaction.
Understanding the Reactants: HCl and NaOH
Before diving into the reaction itself, let's briefly examine the properties of the individual reactants: hydrochloric acid (HCl) and sodium hydroxide (NaOH).
Hydrochloric Acid (HCl)
Hydrochloric acid is a strong acid, meaning it completely dissociates into its ions (H⁺ and Cl⁻) in aqueous solution. This complete dissociation is key to understanding its behavior in reactions. The high concentration of H⁺ ions is what gives HCl its acidic properties. It's a highly corrosive substance and is commonly found in industrial applications and as a component of stomach acid.
Sodium Hydroxide (NaOH)
Sodium hydroxide, also known as lye or caustic soda, is a strong base. Similar to HCl, it completely dissociates in aqueous solution, yielding Na⁺ and OH⁻ ions. The high concentration of OH⁻ ions is responsible for its alkaline properties. NaOH is a highly reactive substance used in various industrial processes, including soap making and drain cleaning.
The Balanced Molecular Equation
The reaction between HCl and NaOH produces sodium chloride (NaCl), a salt, and water (H₂O). The balanced molecular equation represents the overall reaction without explicitly showing the ionic species:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This equation tells us that one mole of hydrochloric acid reacts with one mole of sodium hydroxide to produce one mole of sodium chloride and one mole of water. The (aq) denotes aqueous solutions (dissolved in water), while (l) indicates liquid water.
The Complete Ionic Equation
To understand the reaction at a deeper level, we need to consider the complete ionic equation. This equation shows all the ions present in the solution before and after the reaction. Since both HCl and NaOH are strong electrolytes, they fully dissociate into their respective ions:
H⁺(aq) + Cl⁻(aq) + Na⁺(aq) + OH⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
This equation shows all the ions participating in the reaction, both reactants and products. Notice that sodium (Na⁺) and chloride (Cl⁻) ions appear on both sides of the equation.
The Net Ionic Equation: The Essence of the Reaction
The net ionic equation focuses on the species that actually participate in the chemical change. It eliminates the spectator ions, which are ions that appear unchanged on both sides of the complete ionic equation. In this case, Na⁺ and Cl⁻ are spectator ions. They are present in the solution but don't directly participate in the reaction's core process.
By removing the spectator ions, we arrive at the net ionic equation:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This simple yet powerful equation reveals the fundamental nature of the acid-base neutralization reaction: hydrogen ions (H⁺) from the acid react with hydroxide ions (OH⁻) from the base to form water. This is the essence of the reaction, irrespective of the specific strong acid and strong base used.
Significance of the Net Ionic Equation
The net ionic equation offers several advantages:
- Simplicity and Clarity: It simplifies the representation of the reaction, focusing on the crucial chemical changes.
- Understanding Reaction Mechanism: It highlights the fundamental process of neutralization – the combination of H⁺ and OH⁻ to form water.
- Predicting Reactions: It allows for the prediction of similar reactions between other strong acids and strong bases. The net ionic equation will always be the same for such reactions.
- Stoichiometric Calculations: It simplifies stoichiometric calculations as it only considers the reacting species.
Applications of the HCl and NaOH Reaction
The reaction between HCl and NaOH, or similar strong acid-strong base reactions, has numerous applications across various fields:
- Titrations: This reaction forms the basis of acid-base titrations, a crucial technique in analytical chemistry for determining the concentration of unknown solutions.
- pH Control: This reaction is used to adjust the pH of solutions in various chemical processes and industrial applications. Adding either HCl or NaOH allows for precise pH control.
- Chemical Synthesis: Neutralization reactions are often used in chemical synthesis as a way to remove excess acid or base from a reaction mixture.
- Wastewater Treatment: The reaction is used to neutralize acidic or basic wastewater before discharge, protecting the environment.
- Everyday Applications: This reaction indirectly plays a role in many everyday products and processes, from antacids (neutralizing stomach acid) to soap making (saponification).
Beyond Strong Acids and Bases: Weak Acids and Bases
It's important to note that the simplicity of the net ionic equation (H⁺(aq) + OH⁻(aq) → H₂O(l)) is specific to reactions involving strong acids and strong bases. When weak acids or weak bases are involved, the complete dissociation assumption no longer holds. Weak acids and bases only partially dissociate, and their equilibrium needs to be considered when writing the net ionic equation. This makes the net ionic equations for reactions involving weak acids or bases more complex.
Practical Considerations and Safety Precautions
When working with HCl and NaOH, it's crucial to remember that both are corrosive substances. Appropriate safety measures must always be followed:
- Eye protection: Safety goggles are mandatory.
- Protective clothing: Lab coats and gloves should be worn.
- Careful handling: Avoid direct contact with skin and eyes.
- Proper disposal: Waste solutions must be disposed of according to safety regulations.
Conclusion
The reaction between HCl and NaOH, and its net ionic equation, provides a fundamental understanding of acid-base chemistry. The simplicity of the net ionic equation (H⁺(aq) + OH⁻(aq) → H₂O(l)) for strong acid-strong base reactions highlights the core process of neutralization. This understanding is crucial for various applications, from analytical chemistry to industrial processes. However, it's equally crucial to remember the safety precautions necessary when handling these corrosive chemicals. By understanding both the chemical principles and the safety protocols, one can confidently apply this knowledge in various contexts.
Latest Posts
Latest Posts
-
How Many Valence Electrons In Br
Mar 19, 2025
-
A Flywheel In The Form Of A Uniformly Thick Disk
Mar 19, 2025
-
What Is The Inverse Of 2 5
Mar 19, 2025
-
What Is The Absolute Value Of 4
Mar 19, 2025
-
Find The Instantaneous Rate Of Change
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Net Ionic Equation For Hcl And Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
