How Many Valence Electrons In Br
listenit
Mar 19, 2025 · 5 min read
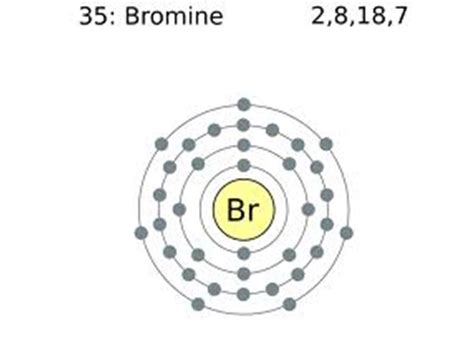
Table of Contents
How Many Valence Electrons Does Bromine (Br) Have? A Deep Dive into Atomic Structure and Chemical Bonding
Bromine (Br), a fascinating halogen element, plays a crucial role in various chemical reactions and industrial processes. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its chemical behavior and reactivity. This comprehensive guide delves into the intricacies of bromine's atomic structure, explaining how to determine its valence electrons and highlighting their significance in chemical bonding and compound formation.
Understanding Valence Electrons: The Key to Chemical Reactivity
Before focusing on bromine specifically, let's establish a fundamental understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the most loosely bound to the nucleus and, therefore, are the ones most likely to participate in chemical bonding. The number of valence electrons dictates an element's reactivity and the types of bonds it can form. Atoms tend to react in ways that achieve a stable electron configuration, often resembling the noble gas configuration (eight valence electrons, except for helium with two). This principle is known as the octet rule.
Determining the Number of Valence Electrons in Bromine
Bromine's atomic number is 35, meaning it has 35 protons and 35 electrons in a neutral atom. To determine the number of valence electrons, we need to look at its electron configuration. The electron configuration of bromine is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁵.
This configuration tells us the distribution of electrons across different energy levels and subshells. The outermost shell for bromine is the fourth shell (n=4), which contains the 4s and 4p subshells. Adding the electrons in these subshells: 4s² + 4p⁵ = 7 electrons.
Therefore, bromine (Br) has 7 valence electrons.
Visualizing Bromine's Electron Configuration
Imagine the electrons arranged in concentric shells around the nucleus. The first shell can hold up to 2 electrons, the second up to 8, the third up to 18, and so on. For bromine:
- Shell 1: 2 electrons (1s²)
- Shell 2: 8 electrons (2s²2p⁶)
- Shell 3: 18 electrons (3s²3p⁶3d¹⁰)
- Shell 4: 7 electrons (4s²4p⁵) – These are the valence electrons.
This visual representation clearly shows that the outermost shell of bromine possesses 7 electrons, emphasizing its valence electron count.
The Significance of Bromine's 7 Valence Electrons
Bromine's seven valence electrons are the key to understanding its chemical properties and behavior. Having seven valence electrons means bromine is one electron short of achieving a stable octet configuration (eight valence electrons) like the noble gas krypton. This drives its reactivity. To achieve stability, bromine readily gains one electron, forming a bromide ion (Br⁻) with a complete octet.
Bromine's Chemical Bonding
Bromine's strong tendency to gain an electron leads to the formation of various chemical bonds:
-
Ionic Bonds: Bromine readily forms ionic bonds with electropositive elements (metals) that easily lose electrons. The metal atom donates an electron to bromine, forming a positively charged metal cation and a negatively charged bromide anion (Br⁻). The electrostatic attraction between these oppositely charged ions forms the ionic bond. Examples include sodium bromide (NaBr) and potassium bromide (KBr).
-
Covalent Bonds: Bromine also participates in covalent bonds with other nonmetals. In covalent bonding, bromine shares one electron pair with another atom to achieve a stable octet. This results in the formation of molecules like hydrogen bromide (HBr) and bromine itself (Br₂). In Br₂, two bromine atoms share one electron pair to complete their octets.
-
Polar Covalent Bonds: When bromine bonds with other atoms of different electronegativity, polar covalent bonds are formed. Electronegativity is the ability of an atom to attract electrons within a bond. Bromine is more electronegative than hydrogen, so in HBr, the shared electron pair is pulled closer to the bromine atom, creating a dipole moment.
Applications of Bromine and its Compounds
The unique chemical properties stemming from bromine's seven valence electrons make it and its compounds crucial in numerous applications:
-
Flame Retardants: Organobromine compounds are frequently used as flame retardants in plastics, textiles, and electronic devices. These compounds interfere with the combustion process, slowing the spread of fire.
-
Water Treatment: Bromine and its compounds are utilized in water purification and disinfection, effectively eliminating bacteria and other harmful microorganisms.
-
Agriculture: Certain bromine compounds act as effective fumigants, controlling pests and soilborne diseases in agriculture.
-
Medicine: Bromine-containing compounds have been employed in various medicinal applications, including sedatives and anticonvulsants. However, their use is now limited due to the potential for side effects.
-
Photography: Silver bromide (AgBr) is a crucial component in photographic film and paper, responsible for the light-sensitive properties.
Environmental Considerations and Safety Precautions
While bromine and its compounds have many valuable applications, their environmental impact and safety must be carefully considered. Some organobromine compounds are persistent organic pollutants (POPs), accumulating in the environment and potentially causing harm to human health and ecosystems. Handling bromine and its compounds requires appropriate safety precautions, including proper ventilation and protective equipment, to prevent exposure to its toxic vapors.
Conclusion: The Importance of Valence Electrons in Understanding Bromine's Chemistry
The number of valence electrons dictates an element's chemical properties and reactivity. Bromine, with its seven valence electrons, demonstrates a strong tendency to gain one electron to achieve a stable octet, resulting in its diverse range of chemical bonding capabilities and applications. Understanding the concept of valence electrons and its implications is crucial for comprehending bromine's role in various chemical reactions and its significant impact across various fields, from flame retardants to medicine and photography. However, responsible use and handling are crucial due to potential environmental and health concerns. This detailed exploration of bromine's valence electrons provides a solid foundation for further study into its fascinating chemistry and diverse applications.
Latest Posts
Latest Posts
-
Molar Volume Of Gas At Stp
Mar 20, 2025
-
Which Of The Following Is A Transition Metal
Mar 20, 2025
-
How Many Valence Electrons Are In Zinc
Mar 20, 2025
-
32 Is 40 Percent Of What Number
Mar 20, 2025
-
How Long Does It Take To Get Saturn
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Br . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
