Molar Volume Of Gas At Stp
listenit
Mar 20, 2025 · 5 min read
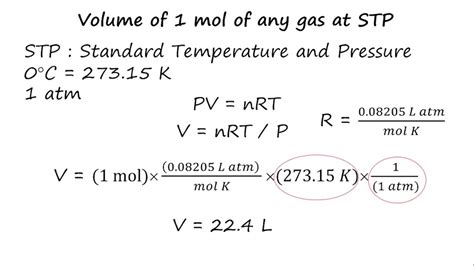
Table of Contents
Molar Volume of Gas at STP: A Comprehensive Guide
The molar volume of a gas at standard temperature and pressure (STP) is a fundamental concept in chemistry, crucial for understanding gas behavior and performing stoichiometric calculations. This comprehensive guide will delve into the definition, calculation, implications, and deviations from the ideal gas law, providing a thorough understanding of this essential concept.
What is STP?
Before delving into molar volume, we must define standard temperature and pressure (STP). While there have been historical variations, the most widely accepted definition today is:
- Temperature: 273.15 Kelvin (0° Celsius or 32° Fahrenheit)
- Pressure: 1 atmosphere (atm) or 101.325 kilopascals (kPa) This is equivalent to 760 mmHg or 760 torr.
These conditions represent a readily reproducible and easily understood benchmark for comparing gas behavior.
Defining Molar Volume
Molar volume is defined as the volume occupied by one mole of a substance. For gases, under specific conditions like STP, this volume is remarkably consistent for ideal gases. The molar volume of an ideal gas at STP is approximately 22.4 liters per mole (L/mol). This means that one mole of any ideal gas at STP will occupy a volume of approximately 22.4 liters.
Ideal Gas Law and Molar Volume
The ideal gas law forms the foundation for understanding molar volume. The equation is:
PV = nRT
Where:
- P represents pressure
- V represents volume
- n represents the number of moles
- R represents the ideal gas constant (0.0821 L·atm/mol·K)
- T represents temperature in Kelvin
To calculate the molar volume (V<sub>m</sub>), we can rearrange the ideal gas law:
V<sub>m</sub> = V/n = RT/P
At STP (T = 273.15 K, P = 1 atm), the molar volume (V<sub>m</sub>) is:
V<sub>m</sub> = (0.0821 L·atm/mol·K × 273.15 K) / 1 atm ≈ 22.4 L/mol
This calculation demonstrates the origin of the commonly used value of 22.4 L/mol for the molar volume of an ideal gas at STP.
Applications of Molar Volume
The concept of molar volume at STP finds extensive applications in various chemical calculations and analyses, including:
1. Stoichiometric Calculations:
Molar volume provides a crucial link between the volume and the number of moles of a gas involved in a chemical reaction. Knowing the volume of a gas at STP allows for the calculation of the number of moles, and vice versa. This is essential for solving stoichiometry problems involving gaseous reactants and products.
Example: Consider the combustion of methane (CH₄):
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g)
If 11.2 L of methane react completely at STP, how many moles of carbon dioxide are produced?
Since 1 mole of CH₄ occupies 22.4 L at STP, 11.2 L of CH₄ represents 0.5 moles (11.2 L / 22.4 L/mol). According to the stoichiometry of the reaction, 0.5 moles of CH₄ produce 0.5 moles of CO₂.
2. Determining Gas Density:
Gas density (ρ) is defined as mass per unit volume. Using molar volume and molar mass (M), we can calculate gas density at STP:
ρ = M/V<sub>m</sub>
For example, the molar mass of oxygen (O₂) is 32 g/mol. Therefore, the density of oxygen at STP is approximately:
ρ = 32 g/mol / 22.4 L/mol ≈ 1.43 g/L
3. Gas Law Applications:
Molar volume serves as a reference point for understanding gas behavior under various conditions. By comparing the volume occupied by a gas under different conditions to its molar volume at STP, insights can be gained about the influence of pressure and temperature on gas volume.
4. Environmental and Industrial Applications:
In environmental science, molar volume is crucial for calculating the amount of pollutants released into the atmosphere. In industrial settings, it's used in process optimization, managing gas flow rates, and designing chemical reactors.
Deviations from Ideal Gas Law and Molar Volume
It's crucial to remember that the 22.4 L/mol value is an approximation based on the ideal gas law. Real gases exhibit deviations from ideal behavior, particularly at high pressures and low temperatures. These deviations arise from:
- Intermolecular forces: Attractive forces between gas molecules cause them to occupy less volume than predicted by the ideal gas law.
- Molecular volume: The finite volume occupied by gas molecules themselves is neglected in the ideal gas law. At high pressures, this volume becomes significant.
These factors cause real gases to have molar volumes slightly different from the ideal value of 22.4 L/mol at STP. The extent of deviation depends on the specific gas and the conditions. The van der Waals equation is often used to account for these deviations and provides a more accurate calculation of molar volume for real gases.
Advanced Considerations: The van der Waals Equation
The van der Waals equation is a more realistic model of gas behavior than the ideal gas law. It accounts for intermolecular forces (represented by the constant 'a') and the volume occupied by the gas molecules themselves (represented by the constant 'b'). The equation is:
(P + a(n/V)²)(V - nb) = nRT
Where:
- a and b are van der Waals constants specific to each gas.
The van der Waals equation provides a more accurate calculation of molar volume, particularly for gases at high pressures or low temperatures where deviations from ideal behavior are significant. However, solving the van der Waals equation directly for molar volume is more complex than using the ideal gas law. Iterative methods or numerical techniques are often employed.
Conclusion: The Importance of Molar Volume
The molar volume of a gas at STP, while an approximation based on the ideal gas law, remains a fundamental concept in chemistry. It provides a convenient and readily usable tool for various stoichiometric calculations, density determinations, and understanding gas behavior. While real gases deviate from ideal behavior, understanding the limitations of the ideal gas law and employing more sophisticated models like the van der Waals equation allows for more accurate estimations of molar volume under non-ideal conditions. The application of molar volume extends to diverse fields, highlighting its significance in both theoretical and practical aspects of chemistry. Its consistent use in various calculations underscores its enduring importance in the scientific community. Continued understanding of this concept is vital for advancements in related scientific disciplines.
Latest Posts
Latest Posts
-
60 Of What Number Is Equal To 30
Mar 20, 2025
-
Is Arcsin The Same As Csc
Mar 20, 2025
-
How Much Is 1 4 Of A Pound
Mar 20, 2025
-
29 Degrees Celsius Is What In Fahrenheit
Mar 20, 2025
-
How Many Valence Electrons Are In Iodine
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Molar Volume Of Gas At Stp . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
