Molar Concentration Of Concentrated Sulfuric Acid
listenit
Mar 24, 2025 · 5 min read
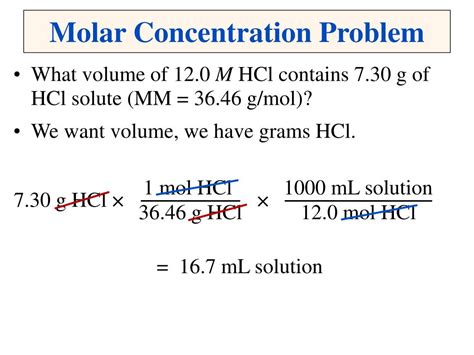
Table of Contents
Molar Concentration of Concentrated Sulfuric Acid: A Deep Dive
Sulfuric acid (H₂SO₄), a cornerstone chemical in countless industrial processes, is often encountered in its concentrated form. Understanding the molar concentration of this concentrated acid is crucial for safe and effective handling, dilution, and application in various contexts. This article will delve into the intricacies of concentrated sulfuric acid's molarity, exploring its variations, calculation methods, and safety precautions.
Understanding Molarity and Concentrated Sulfuric Acid
Molarity (M), a fundamental concept in chemistry, defines the concentration of a solute in a solution. It's expressed as the number of moles of solute per liter of solution. For sulfuric acid, this means the number of moles of H₂SO₄ molecules present in one liter of the concentrated acid solution.
Concentrated sulfuric acid, typically found in laboratories and industrial settings, isn't a precisely defined concentration. The concentration varies slightly depending on the manufacturer and the specific production batch. However, it commonly ranges from 95% to 98% by weight (w/w). This percentage represents the mass of sulfuric acid relative to the total mass of the solution. The remaining percentage is water.
Calculating the Molar Concentration
To determine the molar concentration of a specific batch of concentrated sulfuric acid, we need the percentage by weight and the density of that particular batch. The density, typically expressed in grams per milliliter (g/mL) or kilograms per liter (kg/L), relates the mass of the solution to its volume.
Here's a step-by-step guide on how to calculate the molar concentration:
Step 1: Determine the Percentage by Weight and Density
This information is usually provided by the manufacturer on the product's label or safety data sheet (SDS). Let's assume, for example, we have a batch with a 96% w/w concentration and a density of 1.84 g/mL.
Step 2: Calculate the Mass of Sulfuric Acid in 1 Liter of Solution
- We know that 1 liter (L) equals 1000 milliliters (mL).
- The density is 1.84 g/mL, meaning 1 mL weighs 1.84 g.
- Therefore, 1 L of the solution weighs 1.84 g/mL * 1000 mL = 1840 g.
- Since the solution is 96% H₂SO₄, the mass of sulfuric acid in 1 L is 1840 g * 0.96 = 1766.4 g.
Step 3: Calculate the Number of Moles of Sulfuric Acid
- First, we need the molar mass of H₂SO₄. This is calculated by summing the atomic masses of its constituent atoms: (2 * 1.008) + 32.07 + (4 * 16.00) = 98.08 g/mol.
- The number of moles of H₂SO₄ in 1 L of the solution is then calculated as: 1766.4 g / 98.08 g/mol = 18.01 moles.
Step 4: Determine the Molar Concentration
- Since we have 18.01 moles of H₂SO₄ in 1 L of solution, the molar concentration (M) is 18.01 M.
Therefore, the molar concentration of this specific batch of concentrated sulfuric acid is approximately 18.01 M. Remember that this calculation is based on our example values. You must use the specific percentage by weight and density provided for your batch of concentrated sulfuric acid.
Variations in Concentration and Their Implications
The slight variations in the concentration of concentrated sulfuric acid, as mentioned earlier, can have implications in various applications:
- Chemical Reactions: The exact molarity influences reaction rates and yields. A deviation in concentration can affect the stoichiometry of a reaction, leading to unexpected outcomes.
- Titrations: Accurate molarity is essential for precise titrations, where the concentration of an unknown solution is determined using a solution of known concentration.
- Electrolyte Solutions: In electrochemical applications, the precise molarity determines the conductivity and electrochemical behavior of the solution.
- Industrial Processes: Variations in concentration can directly impact the efficiency and safety of industrial processes.
Safety Precautions When Handling Concentrated Sulfuric Acid
Concentrated sulfuric acid is a highly corrosive and hazardous substance. Extreme caution must be exercised during handling, storage, and disposal:
- Eye Protection: Always wear appropriate eye protection, such as safety goggles or a face shield.
- Protective Clothing: Wear acid-resistant gloves, a lab coat, and closed-toe shoes.
- Ventilation: Work in a well-ventilated area or under a fume hood to minimize exposure to acid fumes.
- Dilution: Always add the acid to water slowly and carefully, never the other way around. Adding water to acid can cause a violent exothermic reaction, potentially leading to splashes and burns.
- Spills: In case of spills, immediately neutralize the acid with a suitable base, such as sodium bicarbonate, and follow proper cleanup procedures.
- Storage: Store concentrated sulfuric acid in a tightly sealed, acid-resistant container in a cool, dry place, away from incompatible materials.
- Disposal: Dispose of concentrated sulfuric acid according to local regulations and guidelines.
Applications of Concentrated Sulfuric Acid
Concentrated sulfuric acid's high reactivity and dehydrating properties make it a versatile chemical used across a wide range of industries:
- Fertilizer Production: A major application is in the production of phosphate fertilizers.
- Petroleum Refining: Used in alkylation processes to produce high-octane gasoline.
- Chemical Synthesis: Serves as a catalyst and dehydrating agent in numerous chemical reactions.
- Metal Processing: Employed in pickling and cleaning of metals.
- Battery Manufacturing: A key component in lead-acid batteries.
- Textile Industry: Used in various processes, such as dyeing and cleaning.
Conclusion: Accuracy and Safety are Paramount
The accurate determination of the molar concentration of concentrated sulfuric acid is crucial for its safe and effective use across numerous applications. While the exact concentration may vary slightly depending on the manufacturer and batch, understanding the calculation method allows for precise control and adaptation in various settings. However, safety should always be the paramount concern when handling this highly corrosive and hazardous chemical. Strict adherence to safety protocols and guidelines is essential to prevent accidents and injuries. Remember, always consult the safety data sheet (SDS) for the specific batch you are working with for the most accurate information on concentration, handling, and safety procedures. The information provided in this article should be considered supplementary to, and not a replacement for, the information provided on the SDS.
Latest Posts
Latest Posts
-
How Do You Graph X 1
Mar 27, 2025
-
What Is The Gcf Of 10 And 12
Mar 27, 2025
-
20 Percent Of What Number Is 40
Mar 27, 2025
-
1 2 Divided By Root 3 Over 2
Mar 27, 2025
-
What Element Is The Most Electronegative
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Molar Concentration Of Concentrated Sulfuric Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
