What Element Is The Most Electronegative
listenit
Mar 27, 2025 · 5 min read
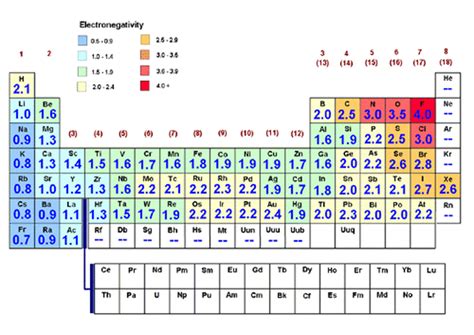
Table of Contents
What Element is the Most Electronegative? Understanding Electronegativity and its Implications
Electronegativity, a fundamental concept in chemistry, dictates how strongly an atom attracts electrons within a chemical bond. Understanding electronegativity is crucial for predicting molecular polarity, bond strength, and the overall reactivity of compounds. While many elements exhibit electronegativity, one stands out as the champion: fluorine. This article delves deep into the concept of electronegativity, explores why fluorine reigns supreme, and examines the implications of this property across various chemical contexts.
Understanding Electronegativity: A Deeper Dive
Electronegativity isn't a directly measurable property like mass or charge. Instead, it's a relative measure, typically represented by a dimensionless number on various scales, most notably the Pauling scale. This scale, developed by Linus Pauling, assigns fluorine a value of 4.0, setting the benchmark for other elements. The higher the electronegativity value, the stronger the atom's pull on bonding electrons.
Several factors influence an atom's electronegativity:
1. Nuclear Charge: The Strong Pull of the Nucleus
A higher nuclear charge signifies a stronger positive attraction exerted by the nucleus on electrons. This increased pull naturally enhances the atom's electronegativity. As you move across a period in the periodic table (left to right), the nuclear charge increases, leading to a corresponding increase in electronegativity.
2. Atomic Radius: Distance Matters
The distance between the nucleus and the valence electrons (the outermost electrons involved in bonding) significantly impacts electronegativity. A smaller atomic radius means the valence electrons are closer to the positively charged nucleus, resulting in a stronger attraction and thus higher electronegativity. As you move down a group in the periodic table, atomic radius increases, leading to a decrease in electronegativity.
3. Shielding Effect: Inner Electrons' Influence
Inner electrons (core electrons) shield the valence electrons from the full positive charge of the nucleus. This shielding effect reduces the net positive charge experienced by the valence electrons, thereby decreasing the atom's electronegativity. The more inner electrons present, the greater the shielding effect.
4. Electron Configuration: Stability and Electronegativity
Atoms strive for stability, often achieved by having a full valence shell (eight electrons, except for hydrogen and helium). Atoms with nearly complete valence shells exhibit higher electronegativity as they strongly attract electrons to fill their outermost shell and achieve stability.
Why Fluorine is the Most Electronegative Element
Fluorine's exceptional electronegativity is a consequence of the interplay of the factors discussed above:
- High Nuclear Charge: Fluorine possesses a relatively high nuclear charge for its period.
- Small Atomic Radius: Fluorine boasts a remarkably small atomic radius. Its valence electrons are exceptionally close to the nucleus, experiencing a strong electrostatic pull.
- Minimal Shielding Effect: Fluorine has only two inner electrons, providing minimal shielding for its valence electrons. This allows the nucleus to exert a nearly unshielded attraction on the valence electrons.
- Near-Complete Valence Shell: Fluorine needs only one more electron to achieve a stable octet electron configuration. This intense desire for an additional electron significantly boosts its electronegativity.
The combined effect of these factors results in fluorine's exceptionally high electronegativity, making it the most electronegative element on the periodic table.
Electronegativity Differences and Bond Polarity
The difference in electronegativity between two bonded atoms determines the polarity of the bond. A large electronegativity difference leads to a polar covalent bond, where the electrons are unequally shared, creating partial positive (δ+) and partial negative (δ-) charges on the atoms. In extreme cases, the electronegativity difference is so significant that one atom essentially takes the electron from the other, resulting in an ionic bond.
Examples:
- HF (Hydrogen Fluoride): Fluorine's high electronegativity creates a highly polar bond with hydrogen. The electrons are pulled strongly toward fluorine, making it partially negative (δ-), while hydrogen becomes partially positive (δ+).
- NaCl (Sodium Chloride): The electronegativity difference between sodium and chlorine is substantial, leading to the formation of an ionic bond. Chlorine completely takes the electron from sodium, resulting in Na⁺ and Cl⁻ ions.
Implications of Electronegativity Across Chemical Disciplines
Electronegativity's influence extends far beyond simple bond polarity. It plays a crucial role in various aspects of chemistry:
1. Predicting Molecular Geometry and Polarity:
The distribution of electron density in a molecule, influenced by the electronegativities of the constituent atoms, dictates its overall polarity and geometry. This, in turn, influences its physical and chemical properties, such as boiling point, solubility, and reactivity.
2. Acid-Base Chemistry:
Electronegativity is crucial in understanding acid-base behavior. Stronger acids often contain atoms with high electronegativities, enabling them to more effectively donate protons (H⁺).
3. Reactivity and Chemical Reactions:
Electronegativity helps predict the likelihood of chemical reactions. Atoms with high electronegativity are more likely to participate in reactions where electron transfer or sharing is involved.
4. Material Science and Engineering:
The design and synthesis of novel materials often relies on careful consideration of electronegativity. Tailoring the electronegativities of constituent atoms enables the creation of materials with specific electrical, magnetic, and optical properties.
Beyond Fluorine: Other Highly Electronegative Elements
While fluorine reigns supreme, other elements also exhibit high electronegativity:
- Oxygen: Oxygen is the second most electronegative element, exhibiting a strong attraction for electrons.
- Nitrogen: Nitrogen also shows significant electronegativity, contributing to the polarity of many nitrogen-containing molecules.
- Chlorine: Chlorine, a halogen like fluorine, possesses high electronegativity, although less than fluorine.
Conclusion: The Reign of Fluorine and the Importance of Electronegativity
Fluorine's unparalleled electronegativity stems from a unique combination of high nuclear charge, small atomic radius, minimal shielding, and a nearly complete valence shell. This property profoundly impacts the chemical behavior of fluorine and the compounds it forms. Understanding electronegativity is vital for predicting and interpreting a wide range of chemical phenomena, from simple bond polarity to the intricate properties of complex molecules and materials. Its influence extends across diverse fields, underscoring its significance as a fundamental concept in chemistry. Therefore, while other elements display appreciable electronegativity, fluorine remains the undisputed champion, holding the title of the most electronegative element.
Latest Posts
Latest Posts
-
Simplify The Square Root Of 54
Mar 30, 2025
-
Ground State Electron Configuration For Cr
Mar 30, 2025
-
What Is 8 Percent In Decimal
Mar 30, 2025
-
How To Find Range Of Function Algebraically
Mar 30, 2025
-
What Is 1 2 3 4
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Element Is The Most Electronegative . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
