Ground State Electron Configuration For Cr
listenit
Mar 30, 2025 · 6 min read
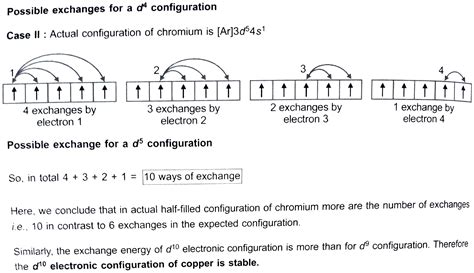
Table of Contents
Ground State Electron Configuration for Chromium: A Deep Dive
Chromium (Cr), element 24 on the periodic table, presents a fascinating exception to Hund's rule and the Aufbau principle when determining its ground state electron configuration. While seemingly straightforward, understanding Chromium's electron configuration requires a deeper understanding of electron-electron interactions and the stability gained through specific orbital arrangements. This article delves into the intricacies of Chromium's electron configuration, exploring the underlying principles, exceptions, and the implications of its unique arrangement.
Understanding Basic Electron Configuration Principles
Before examining Chromium's anomaly, let's review the fundamental principles governing electron configuration:
The Aufbau Principle: Building Up the Atom
The Aufbau principle, German for "building-up" principle, dictates that electrons fill atomic orbitals in order of increasing energy levels. This means electrons first occupy the lowest energy levels available before moving to higher energy levels. The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on.
Hund's Rule: Maximizing Spin Multiplicity
Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This is because electrons, being negatively charged, repel each other. By occupying separate orbitals, they maximize their distance and minimize repulsion, leading to a lower energy state. Each electron in a singly occupied orbital has the same spin (either all spin-up or all spin-down).
Pauli Exclusion Principle: One Electron Per Orbital with Unique Quantum Numbers
The Pauli exclusion principle asserts that no two electrons in an atom can have the same set of four quantum numbers (principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number). This principle ensures that each orbital can hold a maximum of two electrons, each with opposite spins.
The Unexpected Electron Configuration of Chromium
Based solely on the Aufbau principle, one might expect the electron configuration of Chromium (Cr, atomic number 24) to be: 1s²2s²2p⁶3s²3p⁶4s²3d⁴. However, the experimentally determined ground state electron configuration is: 1s²2s²2p⁶3s²3p⁶4s¹3d⁵.
This difference highlights a crucial point: while the Aufbau principle provides a good general guideline, it is not a strict rule, and exceptions exist, particularly for transition metals. The reason for this exception lies in the energy difference between the 4s and 3d orbitals and the stability gained by a half-filled or fully filled d subshell.
The Role of Electron-Electron Repulsion and Exchange Energy
The 4s and 3d orbitals are relatively close in energy. While the 4s orbital is generally lower in energy than the 3d orbital in the Aufbau principle's simplified model, the situation is more nuanced. The increased electron-electron repulsion in the 3d⁴ configuration (four electrons in the 3d subshell) raises its energy. Conversely, the 3d⁵ configuration (five electrons in the 3d subshell, each in a separate orbital with parallel spins) gains significant stability through exchange energy.
Exchange energy is a quantum mechanical phenomenon arising from the indistinguishability of electrons. In a half-filled d subshell, the exchange energy is maximized, leading to a lower overall energy state compared to the 4s²3d⁴ configuration. This extra stabilization overcomes the slight energy difference between the 4s and 3d orbitals, resulting in the observed electron configuration.
Understanding Exchange Energy in Detail
Exchange energy is a consequence of the Pauli exclusion principle and the wave-particle duality of electrons. Because electrons are indistinguishable, their wave functions can be interchanged without changing the overall state of the system. This leads to a reduction in the system's energy – the exchange energy.
The greater the number of parallel spins, the greater the exchange energy. A half-filled or completely filled d subshell maximizes the number of parallel spins and therefore maximizes the exchange energy. This is why a half-filled d subshell (d⁵) or a completely filled d subshell (d¹⁰) is exceptionally stable, often overriding the Aufbau principle's predicted configuration.
Implications of Chromium's Electron Configuration
The unique electron configuration of Chromium has several implications:
-
Chemical Properties: Chromium's electronic structure influences its chemical reactivity and oxidation states. The ability to readily lose the 4s electron and one or more 3d electrons contributes to its diverse oxidation states (+2, +3, +6 being the most common). This makes Chromium a versatile element with a wide range of applications.
-
Magnetic Properties: The presence of unpaired electrons in the 3d subshell makes Chromium paramagnetic, meaning it is attracted to a magnetic field. This property is exploited in various applications involving magnetic materials.
-
Spectroscopic Properties: The electron configuration affects the absorption and emission spectra of Chromium compounds. The transitions between different energy levels of the d electrons give rise to the characteristic colors observed in many chromium compounds.
-
Metallic Bonding: The availability of electrons in both 4s and 3d orbitals contributes to the strong metallic bonding in Chromium, resulting in its high melting point and other characteristic metallic properties.
Distinguishing Between Ground State and Excited State
It's crucial to distinguish between the ground state and excited states. The ground state electron configuration represents the lowest energy state of an atom. Excited states occur when an electron absorbs energy and jumps to a higher energy level. Chromium, in its excited states, could potentially have electron configurations deviating from the ground state configuration (e.g., 4s²3d⁴). However, these are higher energy and less stable.
Experimental Evidence Supporting the 4s¹3d⁵ Configuration
Various spectroscopic techniques and magnetic measurements confirm the 4s¹3d⁵ configuration as the ground state for chromium. These techniques provide experimental evidence supporting the theoretical considerations discussed earlier. For example, magnetic susceptibility measurements indicate the presence of six unpaired electrons, aligning with the 4s¹3d⁵ configuration.
Conclusion: The Exception That Proves the Rule
The ground state electron configuration of Chromium, 1s²2s²2p⁶3s²3p⁶4s¹3d⁵, serves as a compelling example of how exceptions to the Aufbau principle can arise due to the complexities of electron-electron interactions and the significant stability associated with half-filled and fully filled subshells. Understanding this exception is crucial for gaining a comprehensive understanding of atomic structure and chemical behavior, particularly within the transition metal series. The interplay between the Aufbau principle, Hund's rule, and the influence of exchange energy, leads to a more nuanced and accurate picture of electron configuration in atoms. The study of Chromium's electron configuration highlights that the principles are guides, not absolute rules, and the reality often shows a more complex interplay of energetic factors. Further exploration of these concepts opens avenues to understand more complex phenomena in chemistry and material science.
Latest Posts
Latest Posts
-
How Many Radians Is One Revolution
Apr 01, 2025
-
Distance Of Planets From The Sun In Kilometers
Apr 01, 2025
-
How To Calculate Keq From Pka
Apr 01, 2025
-
Graph Of Y 1 X 2 1
Apr 01, 2025
-
How Many Molecules Of Sulfur Trioxide Are In 78 0 Grams
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Ground State Electron Configuration For Cr . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
