How To Calculate Keq From Pka
listenit
Apr 01, 2025 · 6 min read
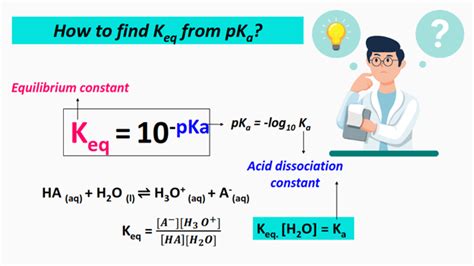
Table of Contents
How to Calculate Keq from pKa: A Comprehensive Guide
The equilibrium constant (Keq) and the acid dissociation constant (pKa) are fundamental concepts in chemistry, particularly in acid-base chemistry and biochemistry. Understanding their relationship and how to calculate Keq from pKa is crucial for various applications, from predicting reaction outcomes to designing buffers. This comprehensive guide will delve into the intricacies of this calculation, providing a step-by-step approach and illustrating it with practical examples.
Understanding Keq and pKa
Before delving into the calculation, let's clarify the definitions of Keq and pKa.
Equilibrium Constant (Keq)
The equilibrium constant (Keq) is a numerical value that describes the ratio of products to reactants at equilibrium for a reversible reaction. A large Keq indicates that the equilibrium favors the formation of products, while a small Keq suggests that the equilibrium favors the reactants. For a generic reversible reaction:
aA + bB ⇌ cC + dD
The Keq expression is:
Keq = ([C]<sup>c</sup>[D]<sup>d</sup>) / ([A]<sup>a</sup>[B]<sup>b</sup>)
where [A], [B], [C], and [D] represent the equilibrium concentrations of the respective species, and a, b, c, and d are their stoichiometric coefficients.
Acid Dissociation Constant (Ka and pKa)
The acid dissociation constant (Ka) specifically describes the equilibrium of an acid's dissociation in water. For a generic weak acid, HA:
HA + H₂O ⇌ H₃O⁺ + A⁻
The Ka expression is:
Ka = ([H₃O⁺][A⁻]) / [HA]
The pKa is simply the negative logarithm (base 10) of Ka:
pKa = -log₁₀(Ka)
A lower pKa value indicates a stronger acid (it dissociates more readily), while a higher pKa value indicates a weaker acid.
Calculating Keq from pKa: The Method
The direct calculation of Keq from pKa hinges on understanding the relationship between the two. The key is recognizing that Ka is itself a specific type of equilibrium constant – the equilibrium constant for the acid dissociation reaction. Therefore, if your overall reaction involves acid dissociation as a component, you can use the Ka (derived from pKa) to determine the overall Keq.
Let's consider a few scenarios:
Scenario 1: The Reaction is Simply Acid Dissociation
If the reaction you are considering is the simple dissociation of an acid, the calculation is straightforward:
-
Calculate Ka from pKa: Use the formula Ka = 10<sup>-pKa</sup>. For example, if pKa = 4.76, then Ka = 10<sup>-4.76</sup> ≈ 1.74 x 10<sup>-5</sup>.
-
Keq = Ka: In this case, the equilibrium constant for the acid dissociation reaction is the Ka value. Therefore, Keq = 1.74 x 10<sup>-5</sup>.
Scenario 2: The Reaction Involves Multiple Equilibria
More complex reactions often involve multiple equilibrium steps. In such cases, you need to combine the equilibrium constants for each individual step to find the overall Keq. The rules for combining equilibrium constants are:
-
For consecutive reactions: If reaction 1 has Keq1 and reaction 2 has Keq2, and they are consecutive steps in a multi-step reaction, then the overall Keq is the product of the individual Keq values: Keq<sub>overall</sub> = Keq1 x Keq2
-
For reactions that add up: If the sum of two reactions gives the overall reaction, the overall Keq is the product of the individual Keq values.
-
For reactions that are reversed: If a reaction is reversed, the Keq of the reversed reaction is the reciprocal of the original Keq: Keq<sub>reversed</sub> = 1/Keq
Let's illustrate this with an example:
Consider a reaction involving two acids, HA and HB, where HA donates a proton to HB:
HA + HB ⇌ A⁻ + H₂B⁺
This reaction can be broken down into two acid dissociation steps:
- HA + H₂O ⇌ H₃O⁺ + A⁻ (Ka1)
- HB + H₂O ⇌ H₃O⁺ + B⁻ (Ka2)
We can reverse reaction 2:
- H₃O⁺ + B⁻ ⇌ HB + H₂O (1/Ka2)
Now we add reactions 1 and 3:
HA + H₂O + H₃O⁺ + B⁻ ⇌ H₃O⁺ + A⁻ + HB + H₂O
Simplifying:
HA + B⁻ ⇌ A⁻ + HB
The Keq for this overall reaction is:
Keq<sub>overall</sub> = Ka1 / Ka2
Since you know the pKa values for HA and HB, you can calculate Ka1 and Ka2 using Ka = 10<sup>-pKa</sup> and then calculate the overall Keq.
Scenario 3: Buffer Solutions and the Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is invaluable when dealing with buffer solutions. It relates pH, pKa, and the ratio of conjugate base to acid:
pH = pKa + log₁₀([A⁻]/[HA])
While this equation doesn't directly give Keq, it allows you to determine the ratio of conjugate base to acid at a specific pH. You can then use this ratio, along with the pKa (and subsequently Ka), to calculate the concentrations of the species involved and substitute these values into the appropriate equilibrium expression to find Keq.
Practical Examples
Let's work through a few concrete examples to solidify our understanding.
Example 1: Simple Acid Dissociation
Acetic acid (CH₃COOH) has a pKa of 4.76. Calculate the Keq for its dissociation in water:
-
Calculate Ka: Ka = 10<sup>-4.76</sup> ≈ 1.74 x 10<sup>-5</sup>
-
Keq = Ka: Keq ≈ 1.74 x 10<sup>-5</sup>
Example 2: Multi-step Equilibrium
Suppose we have two acids, with pKa values of 3.5 and 7.0. Find the overall Keq for the proton transfer reaction between them.
-
Calculate Ka values:
- Ka1 = 10<sup>-3.5</sup> ≈ 3.16 x 10<sup>-4</sup>
- Ka2 = 10<sup>-7.0</sup> ≈ 1.00 x 10<sup>-7</sup>
-
Calculate overall Keq: Keq<sub>overall</sub> = Ka1 / Ka2 = (3.16 x 10<sup>-4</sup>) / (1.00 x 10<sup>-7</sup>) = 3160
Example 3: Using the Henderson-Hasselbalch Equation
A buffer solution is prepared with 0.1 M acetic acid (pKa = 4.76) and 0.2 M sodium acetate. Calculate the pH and then use this to demonstrate how one might indirectly find Keq.
-
Calculate pH: pH = pKa + log₁₀([acetate]/[acetic acid]) = 4.76 + log₁₀(0.2/0.1) ≈ 5.06
-
Determine equilibrium concentrations: The pH tells us the [H3O+] which is 10^-5.06. This allows us to determine the equilibrium concentrations of acetic acid and acetate. This would require an ICE table (Initial, Change, Equilibrium) analysis, and the concentrations found substituted into the Keq expression. The result would be the same Keq as Example 1 because the Henderson-Hasselbalch equation itself is derived from the equilibrium expression for the dissociation of the weak acid.
Conclusion
Calculating Keq from pKa requires a thorough understanding of equilibrium constants, acid dissociation constants, and the relationship between them. Whether the reaction involves simple acid dissociation or more complex equilibria, the fundamental principles remain the same: accurately determining the relevant equilibrium constants for individual steps and then combining them according to the rules of equilibrium calculations. Remember to consider the stoichiometry of the reactions involved. Using tools like the Henderson-Hasselbalch equation can also be useful in indirectly determining the components of the equilibrium expression, ultimately leading to Keq. Mastering this skill is essential for anyone working in fields that involve chemical equilibrium, reaction prediction and designing buffers.
Latest Posts
Latest Posts
-
How Hot Is 42 Degrees Celsius
Apr 02, 2025
-
How To Find The Mass Of Liquid
Apr 02, 2025
-
What Kingdom Does A Human Belong To
Apr 02, 2025
-
Pythagorean Theorem And The Distance Formula
Apr 02, 2025
-
How Long Does It Take Earth To Complete One Rotation
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Keq From Pka . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
