Lewis Structure Of Carbon Monoxide With Formal Charges
listenit
Mar 29, 2025 · 6 min read
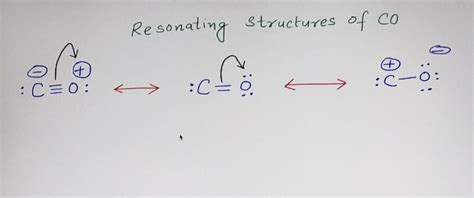
Table of Contents
Lewis Structure of Carbon Monoxide with Formal Charges: A Deep Dive
Carbon monoxide (CO), a simple yet fascinating molecule, presents an excellent case study for understanding Lewis structures and formal charges. Its seemingly straightforward structure belies a subtle complexity that highlights important concepts in chemical bonding and molecular stability. This article provides a comprehensive exploration of the Lewis structure of CO, including a detailed analysis of formal charges and their implications for the molecule's properties.
Understanding Lewis Structures
Before diving into the intricacies of carbon monoxide, let's refresh our understanding of Lewis structures. A Lewis structure, also known as an electron dot diagram, is a visual representation of the valence electrons in a molecule. It shows how atoms are bonded together and how the electrons are distributed around them. These structures are crucial for predicting molecular geometry, polarity, and reactivity. Key components of a Lewis structure include:
- Valence electrons: These are the outermost electrons of an atom, which participate in chemical bonding.
- Bonds: Represented by lines connecting atoms, each line represents a shared pair of electrons (a covalent bond).
- Lone pairs: Represented by pairs of dots, these are electrons not involved in bonding.
The fundamental principle behind drawing Lewis structures is the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight valence electrons (except for hydrogen and helium, which aim for two).
Drawing the Lewis Structure of Carbon Monoxide (CO)
Carbon has four valence electrons and oxygen has six. Therefore, the total number of valence electrons in CO is 10 (4 + 6). Here's a step-by-step process to draw the Lewis structure:
-
Identify the central atom: In CO, both carbon and oxygen are equally electronegative. However, carbon is less electronegative than oxygen, so it's often placed in the center (although in this case, it really doesn't make any significant difference to the final structure).
-
Connect the atoms with single bonds: Begin by connecting the carbon and oxygen atoms with a single covalent bond. This uses two of the ten valence electrons.
-
Distribute the remaining electrons: We have eight electrons left. Complete the octet for the more electronegative atom first (oxygen). This requires six electrons, forming three lone pairs around oxygen.
-
Complete the octet (if possible): Now only two electrons remain. However, this leaves carbon with only two electrons. This is not a stable arrangement!
-
Form multiple bonds: To satisfy the octet rule for both carbon and oxygen, we need to form multiple bonds. Moving a lone pair from the oxygen to form a double bond with carbon leaves both atoms with a filled outer shell (octet).
-
Formal Charges: Now, we calculate the formal charges on each atom.
Calculating Formal Charges
Formal charge is a tool used to assess the distribution of electrons in a molecule. It is calculated using the following formula:
Formal charge = (Valence electrons) - (Non-bonding electrons) - (1/2 Bonding electrons)
Let's calculate the formal charges for each atom in our CO structure with a triple bond:
For Carbon:
- Valence electrons = 4
- Non-bonding electrons = 0
- Bonding electrons = 6 (3 bonds x 2 electrons/bond)
- Formal charge = 4 - 0 - (6/2) = +1
For Oxygen:
- Valence electrons = 6
- Non-bonding electrons = 2 (one lone pair)
- Bonding electrons = 6 (3 bonds x 2 electrons/bond)
- Formal charge = 6 - 2 - (6/2) = +1
Analyzing the Formal Charges and Resonance
The initial triple bond Lewis structure results in formal charges of +1 on both carbon and oxygen. This is not ideal, as we prefer Lewis structures with the lowest possible formal charges. This suggests resonance is at play.
Resonance describes the phenomenon where a molecule can be represented by multiple Lewis structures that differ only in the placement of electrons. These resonance structures are not separate entities; rather, the actual molecule is a hybrid or average of all contributing resonance structures.
In the case of CO, we can also draw a resonance structure with a quadruple bond, though less common. In this resonance structure, the formal charges are also significant and this can be problematic.
Considering a resonance structure with a single bond and two lone pairs on carbon, a triple bond, and one lone pair on oxygen, then the formal charges are: Carbon: 4 - 4 - (2/2) = 0 Oxygen: 6 - 2 - (6/2) = +1 This is still not ideal as it involves non-zero formal charges.
The best representation of CO incorporates the concept of resonance, acknowledging that the true structure is a hybrid between different contributing forms, with partial multiple bond character. This explains the strong bond enthalpy of CO and its relatively short bond length.
Importance of Formal Charges in Understanding Molecular Properties
Formal charges provide insights into several aspects of molecular behavior:
-
Predicting reactivity: Atoms with significant positive formal charges are likely to be more susceptible to nucleophilic attack (attack by electron-rich species). Conversely, atoms with negative formal charges are more prone to electrophilic attack (attack by electron-deficient species).
-
Stability: Structures with minimal formal charges tend to be more stable. This is because charge separation leads to increased energy.
-
Bond polarity: Formal charges help predict bond polarity, which influences the molecule's overall dipole moment and its interactions with other molecules.
In CO, the presence of formal charges, even in the resonance hybrid, indicates a polar molecule with a significant dipole moment despite the formal charges being cancelled out in the hybrid structure.
Beyond Formal Charges: Other Important Considerations for CO
While formal charges are a helpful tool, they are not the sole determinant of molecular properties. Several other factors play a significant role:
-
Electronegativity: Oxygen is more electronegative than carbon, meaning it attracts electrons more strongly. This influences the bond polarity and charge distribution within the molecule, even considering the formal charges.
-
Bond order: The bond order (number of bonds between two atoms) directly relates to bond strength and length. CO's bond order (approximately 3) accounts for its high bond energy and short bond length.
-
Molecular Orbital Theory: For a more accurate and comprehensive description of bonding in CO, molecular orbital theory (MOT) provides a more nuanced understanding than simple Lewis structures. MOT considers the combination of atomic orbitals to form molecular orbitals, leading to a more detailed depiction of electron distribution and bonding.
Conclusion
The Lewis structure of carbon monoxide, while seemingly simple, showcases the complexities of chemical bonding and the importance of resonance. While calculating formal charges helps us understand electron distribution and potential reactivity, it's crucial to remember that this is just one piece of the puzzle. The molecule's true properties emerge from a combination of factors, including resonance, electronegativity differences, bond order, and more sophisticated bonding models like molecular orbital theory. A thorough understanding of these concepts is essential for comprehending the chemical behavior of carbon monoxide and many other molecules. The careful consideration of Lewis structures, including formal charges, is a cornerstone of understanding the world around us at the molecular level.
Latest Posts
Latest Posts
-
What Are 3 Subatomic Particles And Their Charges
Apr 01, 2025
-
How To Find Perimeter With Area Given
Apr 01, 2025
-
What Is The Fraction Of 1
Apr 01, 2025
-
Is Evaporating Water A Physical Change
Apr 01, 2025
-
Elements In The Same Group Have Similar
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Lewis Structure Of Carbon Monoxide With Formal Charges . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
